BDBM50367247 QUININE::Quinamm::Quinsan::cid_3034034
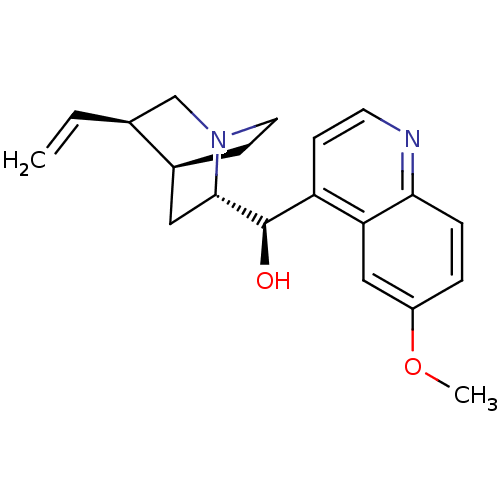
SMILES COc1ccc2c(c1)c(ccn2)[C@H]([C@@H]3C[C@@H]4CC[N@]3C[C@@H]4C=C)O
InChI Key InChIKey=LOUPRKONTZGTKE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 45 hits for monomerid = 50367247
Found 45 hits for monomerid = 50367247
Affinity DataIC50: 10nMAssay Description:Inhibition of human CYP2D6 preincubated for 5 mins followed by NADPH addition and measured after 45 mins by luminescence based microplate reader anal...More data for this Ligand-Target Pair
Affinity DataIC50: 94nMAssay Description:Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D2 expressed in Saccharomyces cerevisiaeMore data for this Ligand-Target Pair
Affinity DataIC50: 280nMAssay Description:TP_TRANSPORTER: inhibition of MPP+ uptake (MPP+: 1 uM) in Xenopus laevis oocytesMore data for this Ligand-Target Pair
Affinity DataIC50: 610nMAssay Description:Inhibition of MAMC O-dealkylation mediated by human Cytochrome P450 2D6 expressed in human lymphoblastoid cell lineMore data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:TP_TRANSPORTER: inhibition of TEA uptake (in the presence of bicarbonate) (TEA: 20 uM) in OCT1-expressing HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 930nMAssay Description:TP_TRANSPORTER: inhibition of TEA uptake in Xenopus laevis oocytesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:TP_TRANSPORTER: inhibition of TEA uptake (in the absence of bicarbonate) (TEA: 20 uM) in OCT1-expressing HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D4 expressed in Saccharomyces cerevisiaeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of P-gp-mediated Rhodamine-123 efflux in human MCF-7/DOX cells assessed as Rhodamine-123 accumulation preincubated for 15 mins followed by...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of human CYP2D6 expressed in Escherichia coli JM109More data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:TP_TRANSPORTER: inhibition of MPP+ uptake (MPP+: 1 uM) in Xenopus laevis oocytesMore data for this Ligand-Target Pair
TargetSolute carrier family 22 member 2(Human)
Anatomisches Institut Der Bayerischen Julius-Maximilians-Universit£T
Curated by ChEMBL
Anatomisches Institut Der Bayerischen Julius-Maximilians-Universit£T
Curated by ChEMBL
Affinity DataKi: 3.40E+3nMAssay Description:TP_TRANSPORTER: inhibition of TEA uptake in Xenopus laevis oocytesMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1A4(Rat)
Kitasato University
Curated by ChEMBL
Kitasato University
Curated by ChEMBL
Affinity DataKi: 3.81E+3nMAssay Description:TP_TRANSPORTER: inhibition of Digoxin uptake in Oatp2-expressing LLC-PK1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.85E+3nMpH: 7.3 T: 2°CAssay Description:The hTREK1 stable cell lines were seeded at a density of 10 000 cells/well in a 12-well plate. Whole-cell membrane currents were amplified using the ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.10E+3nMAssay Description:TP_TRANSPORTER: inhibition of TEA uptake (TEA: 10 uM) in Xenopus laevis oocytesMore data for this Ligand-Target Pair
Affinity DataKi: 4.30E+3nMAssay Description:TP_TRANSPORTER: inhibition of MPP+ uptake in OCT1-expressing HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.40E+3nMAssay Description:Inhibition of binding of Batrachotoxinin [3H]BTX-B to high affinity sites on voltage dependent sodium channels in a vesicular preparation from guinea...More data for this Ligand-Target Pair
Affinity DataKi: 4.60E+3nMAssay Description:Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant)More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D3 expressed in Saccharomyces cerevisiaeMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nMAssay Description:High affinity constant at binding site of human P-Glycoprotein (P-gp) in two-affinity modelMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:TP_TRANSPORTER: inhibition of TEA uptake (in the presence of bicarbonate) (TEA: 20 uM) in OCT2-expressing HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.50E+4nMAssay Description:Inhibition of partially purified cytochrome P450 2D6 1'-hydroxybufuralol formationMore data for this Ligand-Target Pair
Affinity DataKi: 2.10E+4nMAssay Description:Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant)More data for this Ligand-Target Pair
Affinity DataIC50: 2.26E+4nMAssay Description:TP_TRANSPORTER: inhibition of Daunorubicin efflux in NIH-3T3-G185 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.29E+4nMAssay Description:TP_TRANSPORTER: inhibition of TEA uptake in OCT1-expressing HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+4nMAssay Description:TP_TRANSPORTER: inhibition of TEA uptake (in the absence of bicarbonate) (TEA: 20 uM) in OCT2-expressing HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.65E+4nMAssay Description:Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D1 expressed in Saccharomyces cerevisiaeMore data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Human)
Southern Research Institute
Curated by PubChem BioAssay
Southern Research Institute
Curated by PubChem BioAssay
Affinity DataIC50: 5.16E+4nMAssay Description:Inhibition of 4-(4-(dimethylamino)styryl)-N-methylpyridinium uptake at human OCT1 expressed in HEK293 cells by confocal microscopyMore data for this Ligand-Target Pair
Affinity DataKi: 5.50E+4nMAssay Description:TP_TRANSPORTER: inhibition of TEA uptake in Xenopus laevis oocytesMore data for this Ligand-Target Pair
Affinity DataIC50: 7.44E+4nMAssay Description:TP_TRANSPORTER: inhibition of LDS-751 efflux in NIH-3T3-G185 cellsMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1A1(Rat)
Kitasato University
Curated by ChEMBL
Kitasato University
Curated by ChEMBL
Affinity DataKi: 7.67E+4nMAssay Description:TP_TRANSPORTER: inhibition of E217betaG uptake in Oatp1-expressing LLC-PK1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 8.76E+4nMAssay Description:TP_TRANSPORTER: inhibition of Rhodamine 123 efflux in NIH-3T3-G185 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.12E+5nMAssay Description:TP_TRANSPORTER: inhibition of Fluo-3-AM efflux in NIH-3T3-G185 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.35E+5nMAssay Description:Inhibition of human BSEP expressed in fall armyworm sf9 cell plasma membrane vesicles assessed as reduction in vesicle-associated [3H]-taurocholate t...More data for this Ligand-Target Pair
Affinity DataIC50: 1.59E+5nMAssay Description:TP_TRANSPORTER: inhibition of JC-1 efflux in NIH-3T3-G185 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.68E+5nMAssay Description:TP_TRANSPORTER: inhibition of Calcein-AM efflux in NIH-3T3-G185 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.85E+5nMAssay Description:TP_TRANSPORTER: inhibition of Tetramethylrosamine efflux in NIH-3T3-G185 cellsMore data for this Ligand-Target Pair
Affinity DataKd: 3.09E+5nMAssay Description:Binding affinity to human serum albumin by PAMPA methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.30E+5nMAssay Description:Concentration required for 50% inhibition at binding site of human P-Glycoprotein (P-gp) in one-affinity modelMore data for this Ligand-Target Pair
TargetHistidine-rich protein PFHRP-II(malaria parasite P. falciparum)
University of Antwerp
Curated by ChEMBL
University of Antwerp
Curated by ChEMBL
Affinity DataIC50: 7.40E+9nMAssay Description:Inhibition of beta-hematin formation by BHIA assayMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)