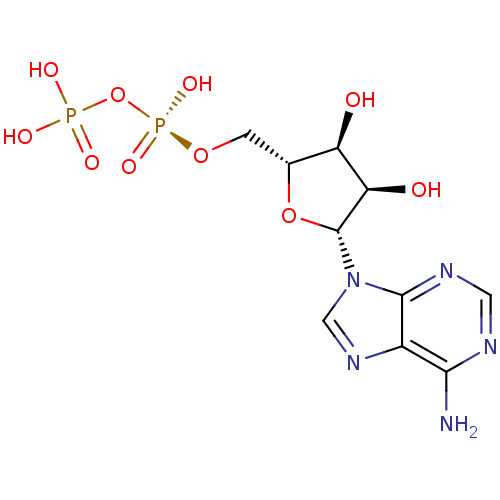
BDBM50368125 ADENOSINE DIPHOSPHATE::ADP
SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O
InChI Key InChIKey=XTWYTFMLZFPYCI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 31 hits for monomerid = 50368125
Found 31 hits for monomerid = 50368125
Affinity DataKi: 0.910nMAssay Description:Inhibition of rat ecto-5'-nucleotidase expressed in Sf9 cells by capillary electrophoresis methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMAssay Description:Inhibition of human CD73 expressed in A-375 cells incubated for 15 mins by malachite green based spectrophotometer assayMore data for this Ligand-Target Pair
Affinity DataEC50: 24nMAssay Description:Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base...More data for this Ligand-Target Pair
Affinity DataEC50: 24nMAssay Description:Agonist activity at human GFP-tagged P2Y1R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresce...More data for this Ligand-Target Pair
Affinity DataEC50: 69nMAssay Description:Antagonist activity against phospholipase C coupled rat P2Y purinoceptor 12 (P2Y12)More data for this Ligand-Target Pair
Affinity DataKi: 88nMAssay Description:Binding affinity to human recombinant CD73 assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataEC50: 100nMAssay Description:Agonist activity at turkey P2Y1 receptor expressed in human 1321N1 cells assessed as increase in intracellular calcium concentration by dual-excitati...More data for this Ligand-Target Pair
Affinity DataKd: 320nMAssay Description:Binding affinity to human truncated HSC70 NBD (1 to 381 residues) by SPR analysisMore data for this Ligand-Target Pair
Affinity DataKd: 324nMAssay Description:Binding affinity to human truncated HSC70 NBD (1 to 381 residues) by SPR analysisMore data for this Ligand-Target Pair
Affinity DataKd: 400nMAssay Description:Displacement of FP-Probe from N-terminal His-tagged full length recombinant human HSET incubated for 2 hrs by fluorescence polarization based competi...More data for this Ligand-Target Pair
Affinity DataEC50: 1.70E+3nMAssay Description:Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas...More data for this Ligand-Target Pair
Affinity DataEC50: 1.70E+3nMAssay Description:Agonist activity at human GFP-tagged P2Y11R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresc...More data for this Ligand-Target Pair
Affinity DataKd: 4.10E+3nMAssay Description:Binding affinity to recombinant human biotinylated N-terminal GST-tagged autophosphorylated TAK1 (1 to 303 residues) fused with TAB1 (437 to 504 resi...More data for this Ligand-Target Pair
Affinity DataIC50: 7.19E+3nMAssay Description:Inhibition of Streptococcus pneumoniae VicK autophosphorylation using compound at non-aggregating concentration after 30 mins in presence of [gamma-3...More data for this Ligand-Target Pair
Affinity DataEC50: 8.00E+3nMAssay Description:Evaluated for agonist activity against phospholipase C coupled P2Y purinoceptor 1 (P2Y1) of turkey erythrocytesMore data for this Ligand-Target Pair
Affinity DataEC50: 1.00E+4nMAssay Description:The compound was evaluated for antagonist activity against recombinant human P2X purinoceptor 1 (P2X1 )More data for this Ligand-Target Pair
Affinity DataEC50: 1.10E+4nMAssay Description:Antagonist activity against recombinant rat P2X purinoceptor 6 (P2X6 )More data for this Ligand-Target Pair
Affinity DataIC50: 1.14E+4nMAssay Description:Displacement of 5-(3-(3-(6-amino-8-(6-iodobenzo[d][1,3]dioxol-5-ylthio)-9H-purin-9-yl)propyl)thioureido)-2-(6-hydroxy-3-oxo-3H-xanthen-9-yl)benzoic a...More data for this Ligand-Target Pair
Affinity DataEC50: 1.80E+4nMAssay Description:Antagonist activity against recombinant rat P2X purinoceptor 5 (P2X5)More data for this Ligand-Target Pair
Affinity DataEC50: 2.10E+4nMAssay Description:Inhibition of BODIPY-AG binding to dog Grp94More data for this Ligand-Target Pair
Affinity DataEC50: 2.80E+4nMAssay Description:The compound was evaluated for antagonist activity against recombinant rat P2X purinoceptor 4 (P2X4) 3 uMMore data for this Ligand-Target Pair
TargetHeat shock protein 75 kDa, mitochondrial(Human)
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
Affinity DataIC50: 5.56E+4nMAssay Description:Displacement of 5-(3-(3-(6-amino-8-(6-iodobenzo[d][1,3]dioxol-5-ylthio)-9H-purin-9-yl)propyl)thioureido)-2-(6-hydroxy-3-oxo-3H-xanthen-9-yl)benzoic a...More data for this Ligand-Target Pair
Affinity DataKd: 6.30E+4nMAssay Description:Binding affinity to recombinant human biotinylated N-terminal GST-tagged non-autophosphorylated TAK1 (1 to 303 residues) fused with TAB1 (437 to 504 ...More data for this Ligand-Target Pair
TargetP2Y purinoceptor 6(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataEC50: 6.50E+4nMAssay Description:Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP productionMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+5nMAssay Description:The compound was evaluated for antagonist activity against recombinant rat P2X purinoceptor 3 (P2X3) at 10 uM, expressed in Xenopus oocytesMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+5nMAssay Description:Evaluated for agonist activity against phospholipase C coupled recombinant human P2Y purinoceptor 2 (P2Y2)More data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+5nMAssay Description:The compound was evaluated for antagonist activity against recombinant human receptor P2X purinoceptor 2 (P2X2 )More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4-kinase type 2-alpha(Human)
Smith Kline & French Research
Curated by ChEMBL
Smith Kline & French Research
Curated by ChEMBL
Affinity DataKi: 1.00E+5nMAssay Description:Binding affinity (Ki) against human phosphatidylinositol 4-kinaseMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4-kinase type 2-alpha(Human)
Smith Kline & French Research
Curated by ChEMBL
Smith Kline & French Research
Curated by ChEMBL
Affinity DataIC50: 1.45E+5nMAssay Description:Inhibitory activity (IC50) against human phosphatidylinositol 4-kinase at the ATP binding siteMore data for this Ligand-Target Pair
Target2-dehydropantoate 2-reductase(Escherichia coli (strain K12))
University Chemical Laboratory
Curated by ChEMBL
University Chemical Laboratory
Curated by ChEMBL
Affinity DataKd: 3.60E+5nMAssay Description:Binding affinity to Escherichia coli KPRMore data for this Ligand-Target Pair
Target2-dehydropantoate 2-reductase(Escherichia coli (strain K12))
University Chemical Laboratory
Curated by ChEMBL
University Chemical Laboratory
Curated by ChEMBL
Affinity DataKi: 1.05E+6nMAssay Description:Inhibition of Escherichia coli KPRMore data for this Ligand-Target Pair