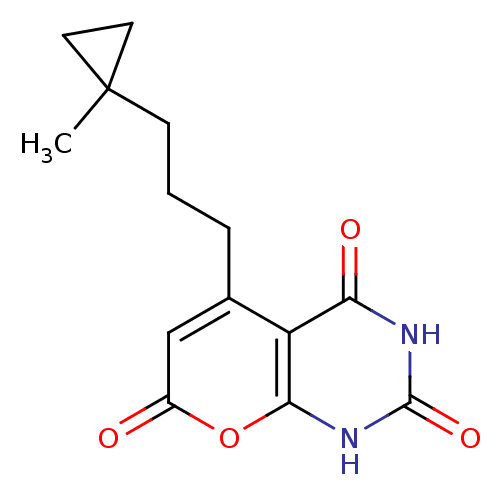
BDBM50384612 CHEMBL2036958
SMILES CC1(CC1)CCCC2=CC(=O)OC3=C2C(=O)NC(=O)N3
InChI Key InChIKey=ARJKMWXLIHZLQZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50384612
Found 8 hits for monomerid = 50384612
Affinity DataEC50: 2nMAssay Description:Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulationMore data for this Ligand-Target Pair
Affinity DataEC50: 6nMAssay Description:Agonist activity at mouse GPR109aMore data for this Ligand-Target Pair
Affinity DataEC50: 8nMAssay Description:Agonist activity at rat GPR109aMore data for this Ligand-Target Pair
Affinity DataEC50: 96nMAssay Description:Agonist activity at human GPR109bMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of human CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of human CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of human CYP3A4More data for this Ligand-Target Pair