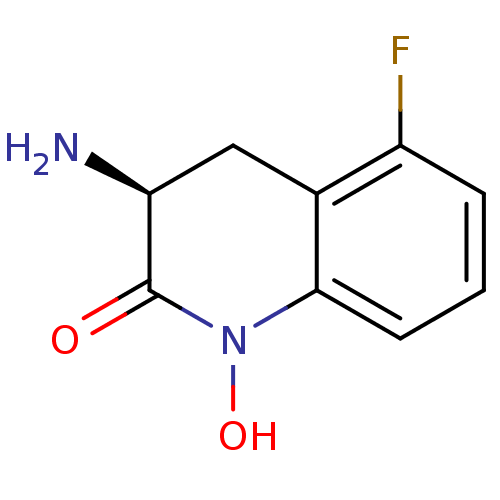
BDBM50386295 CHEMBL2049096
SMILES N[C@H]1Cc2c(F)cccc2N(O)C1=O
InChI Key InChIKey=PYFPGGZHLUSKPB-ZETCQYMHSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50386295
Found 2 hits for monomerid = 50386295
TargetKynurenine/alpha-aminoadipate aminotransferase, mitochondrial(Rattus norvegicus)
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 2.06E+3nMAssay Description:Inhibition of rat recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrsMore data for this Ligand-Target Pair
TargetKynurenine/alpha-aminoadipate aminotransferase, mitochondrial(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 45nMAssay Description:Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrsMore data for this Ligand-Target Pair