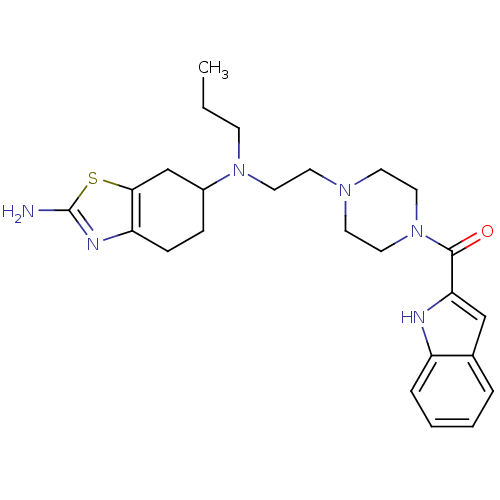
BDBM50392125 CHEMBL2152746
SMILES CCCN(CCN1CCN(CC1)C(=O)c1cc2ccccc2[nH]1)C1CCc2nc(N)sc2C1
InChI Key InChIKey=CPWJJWMVFFZCGJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50392125
Found 3 hits for monomerid = 50392125
Affinity DataKi: 4.60nMAssay Description:Displacement of [3H]spiroperidol from rat cloned dopamine D3 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 114nMAssay Description:Agonist activity at human dopamine D2L receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding after 60 mins by scintillati...More data for this Ligand-Target Pair
Affinity DataKi: 852nMAssay Description:Displacement of [3H]spiroperidol from rat cloned dopamine D2L receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair