BDBM50398668 CHEMBL2178134::US8802674, 256
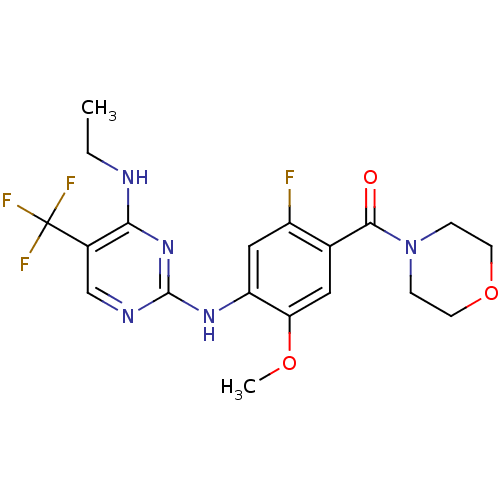
SMILES CCNc1c(cnc(n1)Nc2cc(c(cc2OC)C(=O)N3CCOCC3)F)C(F)(F)F
InChI Key InChIKey=XCFLWTZSJYBCPF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50398668
Found 8 hits for monomerid = 50398668
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Human)
Sichuan University
Curated by ChEMBL
Sichuan University
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of LRRK2 G2019S mutant (unknown origin)More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Human)
Sichuan University
Curated by ChEMBL
Sichuan University
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assayMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Human)
Sichuan University
Curated by ChEMBL
Sichuan University
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Inhibition of LRRK2 G2019S mutant (unknown origin)More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Human)
Sichuan University
Curated by ChEMBL
Sichuan University
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Human)
Sichuan University
Curated by ChEMBL
Sichuan University
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of LRRK2 (unknown origin) phosphorylationMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Human)
Sichuan University
Curated by ChEMBL
Sichuan University
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of autophosphorylation of LRRK2 in human HEK293 cellsMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Human)
Sichuan University
Curated by ChEMBL
Sichuan University
Curated by ChEMBL
Affinity DataIC50: 97nMAssay Description:Inhibition of recombinant human wild type GST-tagged LRRK2 (970 to 2527 residues) preincubated for 30 mins followed by fluorescein-LRRKtide substrate...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Human)
Sichuan University
Curated by ChEMBL
Sichuan University
Curated by ChEMBL
Affinity DataIC50: 97nMAssay Description:Inhibition of recombinant human wild type GST-tagged LRRK2 (970 to 2527 residues) preincubated for 30 mins followed by fluorescein-LRRKtide substrate...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)