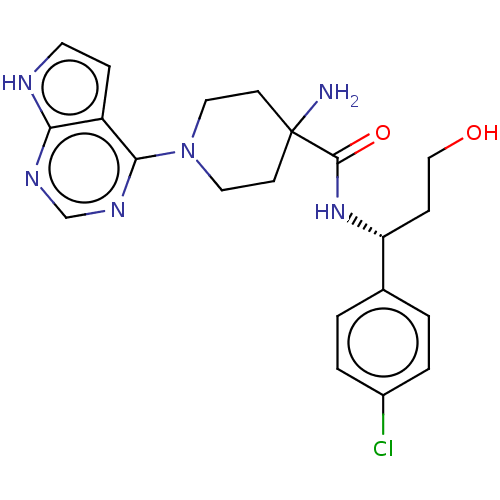
BDBM256836 BDBM50427349::US10654855, Example 15::US11236095, Example 15::US9492453, 15
SMILES NC1(CCN(CC1)c1ncnc2[nH]ccc12)C(=O)N[C@H](CCO)c1ccc(Cl)cc1
InChI Key InChIKey=JDUBGYFRJFOXQC-UHFFFAOYSA-N
Data 16 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 16 hits for monomerid = 256836
Found 16 hits for monomerid = 256836
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant Akt1 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift ass...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMpH: 7.5 T: 2°CAssay Description:This assay detects inhibitors of AKT1 (PKBα) kinase activity using Caliper LabChip LC3000. The Caliper off-chip incubation mobility shift assay...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of recombinant Akt1 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift ass...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of recombinant Akt3 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift ass...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of recombinant Akt2 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift ass...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of recombinant Akt3 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift ass...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of recombinant Akt2 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift ass...More data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Inhibition of Akt1 (unknown origin) expressed in Escherichia coli using peptide substrate after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 56nMAssay Description:Inhibition of recombinant ROCK2 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift as...More data for this Ligand-Target Pair
Affinity DataIC50: 93nMpH: 7.5 T: 2°CAssay Description:This assay detects inhibitors of AKT1 (PKBα) kinase activity using Caliper LabChip LC3000. The Caliper off-chip incubation mobility shift assay...More data for this Ligand-Target Pair
Affinity DataIC50: 93nMAssay Description:For Echo dosing the solvent was 100% DMSO. A master plate was prepared with 40 ul of 10 mM stock from our Primary Liquid Store in quadrant 1 of a Lab...More data for this Ligand-Target Pair
Affinity DataIC50: 93nMAssay Description:Following addition of compound or control to the assay plate, 6p1 peptide mix containing 3 μM substrate (5-FAM-GRPRTSSFAEG-CONH2; CRB) and 40 &#...More data for this Ligand-Target Pair
Affinity DataIC50: 112nMAssay Description:Inhibition of recombinant ROCK2 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift as...More data for this Ligand-Target Pair
Affinity DataIC50: 470nMAssay Description:Inhibition of ROCK1 (unknown origin) using FITC-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human ERG expressed in CHO cells by ionworks assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human ERG expressed in CHO cells by ionworks assayMore data for this Ligand-Target Pair