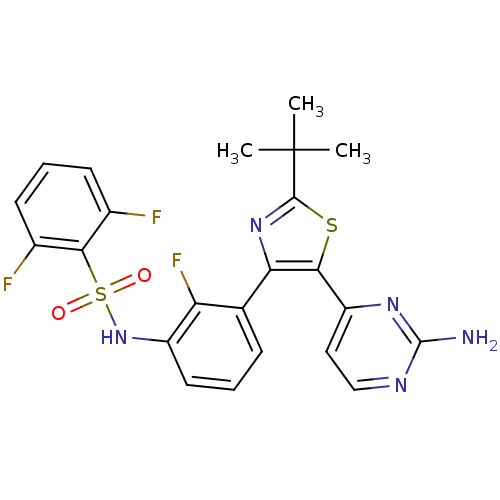
BDBM50428286 DABRAFENIB::GSK2118436A
SMILES CC(C)(C)c1nc(c(s1)c2ccnc(n2)N)c3cccc(c3F)NS(=O)(=O)c4c(cccc4F)F
InChI Key InChIKey=BFSMGDJOXZAERB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 38 hits for monomerid = 50428286
Found 38 hits for monomerid = 50428286
Affinity DataIC50: 0.400nMAssay Description:Inhibition of wild type B-Raf (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of BRAF V600E mutant (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of N-terminal His-tagged human B-Raf kinase domain (448 to 723 residues) expressed in Escherichia coli BL21 (DE3) by Lanthascreen assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibition of B-Raf V600E mutant (unknown origin) in presence of ATP by competitive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.650nMAssay Description:Inhibition of B-RAF V600E mutant (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inhibition of BRAF V600E mutant (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inhibition of BRAF V600E mutant (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 0.700nMAssay Description:Inhibition of N-terminal His-tagged human recombinant B-Raf V600E mutant (448 to 723 residues) expressed in Escherichia coli BL21 (DE3) by Lanthascre...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibition of BRAF V600E mutant (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of B-Raf V600E mutant in human A375 cells assessed as ERK phosphorylation preincubated for 1 hr by Western blot methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant human GST-tagged BRAF V600E mutant expressed in baculovirus expression systemMore data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 3(Human)
Fudan University
Curated by ChEMBL
Fudan University
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human RIPK3 using MBP as substrate by [gamma33-ATP] based radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of BRAF V600E mutant (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of wild-type B-RAF (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of wild type BRAF (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of BRAF V600E mutant in human A375 cells assessed as reduction in ERK phosphorylation by Western blot analysisMore data for this Ligand-Target Pair
TargetRAF proto-oncogene serine/threonine-protein kinase(Human)
Zhengzhou University
Curated by ChEMBL
Zhengzhou University
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of C-Raf (unknown origin) in presence of ATP by competitive binding assayMore data for this Ligand-Target Pair
TargetRAF proto-oncogene serine/threonine-protein kinase(Human)
Zhengzhou University
Curated by ChEMBL
Zhengzhou University
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of wild type C-RAF (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of BRAF (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of recombinant human full length GST-tagged BRAF expressed in baculovirus expression systemMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Competitive binding affinity to BRAF in human A375 cells after 15 mins in presence of ATP analogueMore data for this Ligand-Target Pair
Affinity DataIC50: 9.70nMAssay Description:Inhibition of wild type BRAF (unknown origin) assessed as ADP formation measured for 5 hrs by pyruvate kinase/lactate dehydrogenase coupled assay in ...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of BRAF V600E mutant in human A375 cells assessed as inhibition of ERK phosphorylation measured after 72 hrs by ELISA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Competitive binding affinity to ARAF in human A375 cells after 15 mins in presence of ATP analogueMore data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 2(Human)
Fudan University
Curated by ChEMBL
Fudan University
Curated by ChEMBL
Affinity DataIC50: 77nMAssay Description:Inhibition of human RIPK2 using MBP as substrate by [gamma33-ATP] based radiometric assayMore data for this Ligand-Target Pair
Affinity DataEC50: 82nMAssay Description:Agonist activity at human Pregnane X receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 87nMAssay Description:Transactivation of Gal4-tagged human PXR transfected in human HeLa cells preincubated for 16 hrs followed by luciferin addition and measured after 10...More data for this Ligand-Target Pair
Affinity DataEC50: 87nMAssay Description:Agonist activity at PXR (unknown origin)More data for this Ligand-Target Pair
TargetRAF proto-oncogene serine/threonine-protein kinase(Human)
Zhengzhou University
Curated by ChEMBL
Zhengzhou University
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Competitive binding affinity to CRAF in human A375 cells after 15 mins in presence of ATP analogueMore data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 3(Human)
Fudan University
Curated by ChEMBL
Fudan University
Curated by ChEMBL
Affinity DataIC50: 250nMAssay Description:Inhibition of human full length N-terminal GST-tagged RIPK3 expressed in baculovirus infected sf9 insect cells assessed as reduction in MBP phosphory...More data for this Ligand-Target Pair
Affinity DataEC50: 529nMAssay Description:Inhibition of wild type B-Raf in human MIAPaCa2 cells assessed as reduction in ERK phosphorylation preincubated for 1 hr by Western blot methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibition of Alk5 in TGF-beta-stimulated human HepG2 cells assessed as decrease in Smad2 phosphorylation treated for 45 mins prior to TGF-beta stimu...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Displacement of 6-amino-9-(2-((4-((2-((6-((5-(2,3-dihydrobenzo[b][1,4]dioxine-6-carboxamido)-2-methylphenyl)carbamoyl)quinolin-2-yl)oxy)ethyl)amino)-...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi...More data for this Ligand-Target Pair
Affinity DataIC50: 2.94E+4nMAssay Description:Inhibition of human RIPK5 using MBP as substrate by [gamma33-ATP] based radiometric assayMore data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Human)
Fudan University
Curated by ChEMBL
Fudan University
Curated by ChEMBL
Affinity DataIC50: 5.32E+4nMAssay Description:Inhibition of human RIPK1 using MBP as substrate by [gamma33-ATP] based radiometric assayMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)