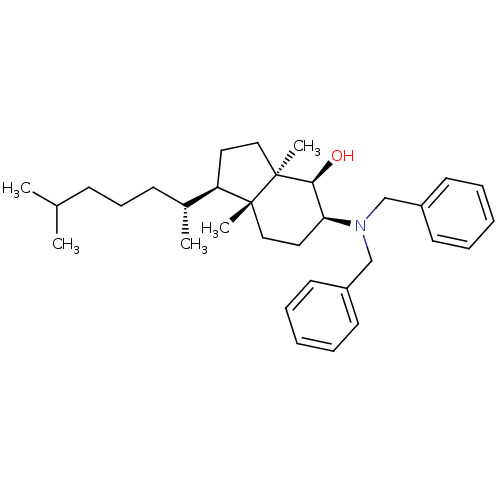
BDBM50430555 CHEMBL2336917
SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@@]2(C)[C@@H](O)[C@H](CC[C@]12C)N(Cc1ccccc1)Cc1ccccc1
InChI Key InChIKey=ITPTXDSAPQUGKF-TUCWNJAESA-N
Data 1 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50430555
Found 1 hit for monomerid = 50430555
Target3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase(Homo sapiens (Human))
Ludwig-Maximilians University
Curated by ChEMBL
Ludwig-Maximilians University
Curated by ChEMBL
Affinity DataIC50: 2.70E+4nMAssay Description:Inhibition of delta8,7-sterol isomerase-mediated cholesterol biosynthesis in human HL60 cells assessed as increase in zymostenol accumulationMore data for this Ligand-Target Pair