BDBM50445062 CHEMBL3098771
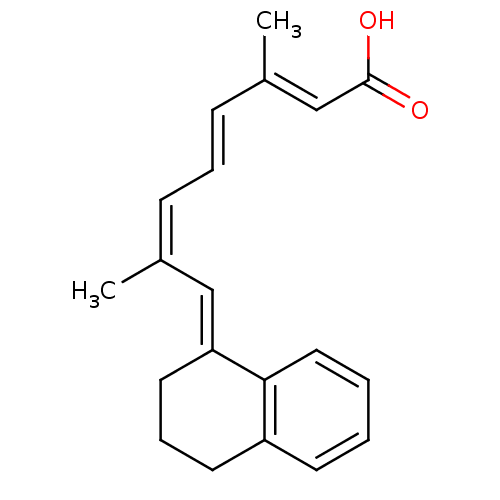
SMILES C/C(=C\C(=O)O)/C=C/C=C(/C)\C=C\1/CCCc2c1cccc2
InChI Key InChIKey=PPGNMFUMZSAZCW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50445062
Found 9 hits for monomerid = 50445062
Affinity DataKd: 29nMAssay Description:Binding affinity to human RXRalpha LBD (T225 to T462 residues) by fluorescence quenching assayMore data for this Ligand-Target Pair
Affinity DataKd: 33nMAssay Description:Binding affinity to human RXR-alpha-ligand binding domain homodimers by fluorescence quenching methodMore data for this Ligand-Target Pair
Affinity DataIC50: 284nMAssay Description:Inhibition of RXRalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 400nMAssay Description:Agonist activity at RXR-alpha in rat R3KE cells infected with oncogene KLF4-ER assessed as inhibition of KLF4-mediated oncogenic transformationMore data for this Ligand-Target Pair
Affinity DataEC50: 820nMAssay Description:Agonist activity at RXRalpha in rat RK3E cells assessed as transcriptional activation by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 820nMAssay Description:Agonist activity at Gal4-fused human RXR-alpha expressed in HEK293 cells assessed as receptor-mediated transcriptional activity treated 24 hrs after ...More data for this Ligand-Target Pair
Affinity DataEC50: 850nMAssay Description:Agonist activity at human GAL4-fused RXRalpha LBD (T225 to T462 residues) expressed in HEK293 cells incubated for 24 hrs by Dual-Glo luciferase assayMore data for this Ligand-Target Pair
Affinity DataKd: 1.33E+3nMAssay Description:Binding affinity to human RXRalpha LBD after 15 mins by isothermal titration calorimetry assayMore data for this Ligand-Target Pair
Affinity DataKd: 1.88E+3nMAssay Description:Agonist activity at human RXR-alpha-ligand binding domain homodimers assessed as coactivator recruitment by measuring GRIP1 binding to receptor by is...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)