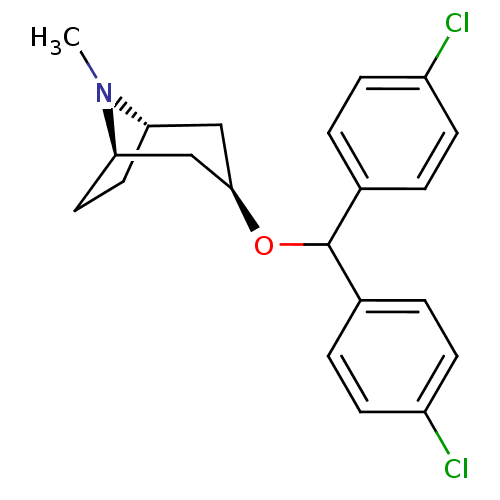
BDBM50453902 CHEMBL3084884
SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OC(c1ccc(Cl)cc1)c1ccc(Cl)cc1)N2C
InChI Key InChIKey=DAAKBMHWKLVSKY-PMOLBWCYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50453902
Found 5 hits for monomerid = 50453902
TargetSodium-dependent dopamine transporter(Rattus norvegicus (rat))
National Institutes Of Health
Curated by ChEMBL
National Institutes Of Health
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Affinity for rat dopamine transporter using [3H]WIN-35428 displacement.More data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Rattus norvegicus (rat))
National Institutes Of Health
Curated by ChEMBL
National Institutes Of Health
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Binding affinity to dopamine transporter (DAT) using [3H]WIN-35428 as a radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 35nMAssay Description:Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement.More data for this Ligand-Target Pair
Affinity DataKi: 1.49E+3nMAssay Description:Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement.More data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Rattus norvegicus (rat))
National Institutes Of Health
Curated by ChEMBL
National Institutes Of Health
Curated by ChEMBL
Affinity DataIC50: 75nMAssay Description:Inhibition of [3H]dopamine uptake in rat caudate putamen.More data for this Ligand-Target Pair