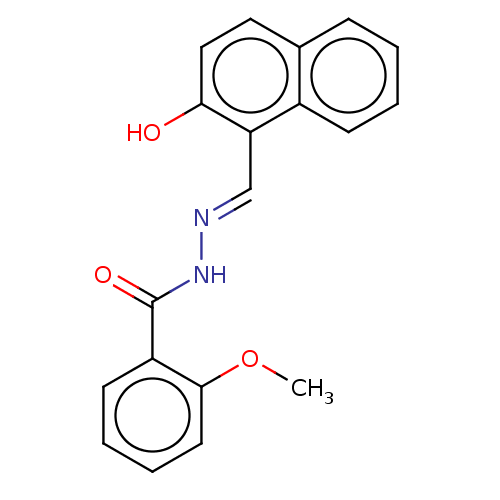
BDBM50484808 CHEMBL1958233::NSC-205497
SMILES COc1ccccc1C(=O)N\N=C\c1c(O)ccc2ccccc12
InChI Key InChIKey=SGIUPSNOFYYWMP-UDWIEESQSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50484808
Found 3 hits for monomerid = 50484808
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of RNA-dependent DNA polymerase activity of Human immunodeficiency virus 1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.13E+4nMAssay Description:Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells assessed as reduction in kynurenine production using L-tryptophan as substrate incubated ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+4nMAssay Description:Inhibition of ribonuclease H activity of HIV1 reverse transcriptase using as poly(dC)-[3H]poly(rG) as substrateMore data for this Ligand-Target Pair