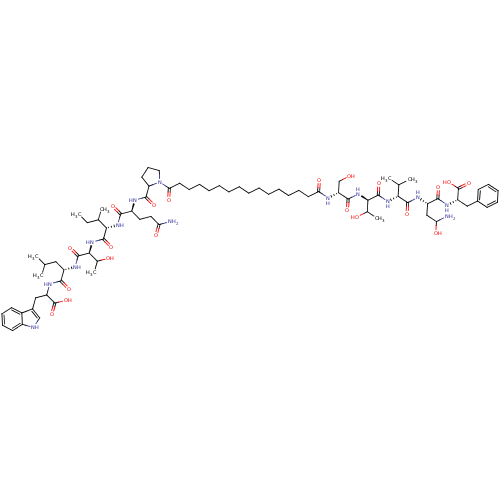BDBM59213 Pepstatin analog, 1
SMILES CCC(C)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)C1CCCN1C(=O)CCCCCCCCCCCCCCC(=O)N[C@H](CO)C(=O)N[C@H](C(C)O)C(=O)N[C@H](C(C)C)C(=O)N[C@@H](CC(N)O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(=O)N[C@@H](C(C)O)C(=O)N[C@@H](CC(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(O)=O
InChI Key InChIKey=BCOLDJXDDMYENJ-DRHARVDYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 59213
Found 1 hit for monomerid = 59213
TargetCapsid scaffolding protein(Human herpesvirus 1 (strain 17) (HHV-1) (Human her...)
Purdue University
Purdue University
Affinity DataKi: 220nM IC50: 350nMpH: 5.52Assay Description:The competitive assay requires two inhibitors to act by a purely competitive mechanism, whereas the binding site of on the inhibitors has been establ...More data for this Ligand-Target Pair