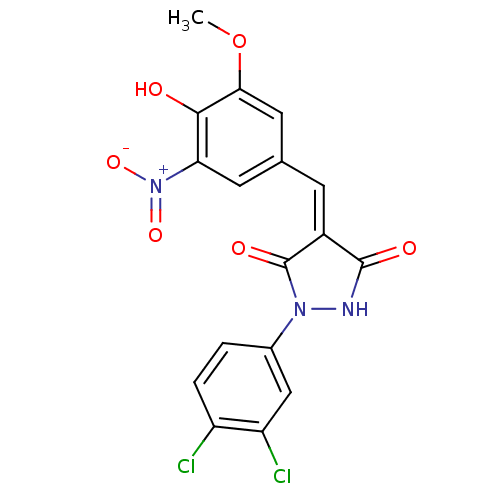
BDBM60937 (4Z)-1-(3,4-dichlorophenyl)-4-(4-hydroxy-3-methoxy-5-nitro-benzylidene)pyrazolidine-3,5-quinone::(4Z)-1-(3,4-dichlorophenyl)-4-(4-hydroxy-3-methoxy-5-nitrobenzylidene)pyrazolidine-3,5-dione::(4Z)-1-(3,4-dichlorophenyl)-4-[(3-methoxy-5-nitro-4-oxidanyl-phenyl)methylidene]pyrazolidine-3,5-dione::(4Z)-1-(3,4-dichlorophenyl)-4-[(4-hydroxy-3-methoxy-5-nitrophenyl)methylidene]pyrazolidine-3,5-dione::MLS001194689::SMR000554908::cid_24793693
SMILES COc1cc(\C=C2\C(=O)NN(C2=O)c2ccc(Cl)c(Cl)c2)cc(c1O)[N+]([O-])=O
InChI Key InChIKey=UXPDAIRBPHPGNP-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 60937
Found 2 hits for monomerid = 60937
TargetHexokinase HKDC1 [W721R](Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 3.25E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetHexokinase HKDC1 [W721R](Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 8.00E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair