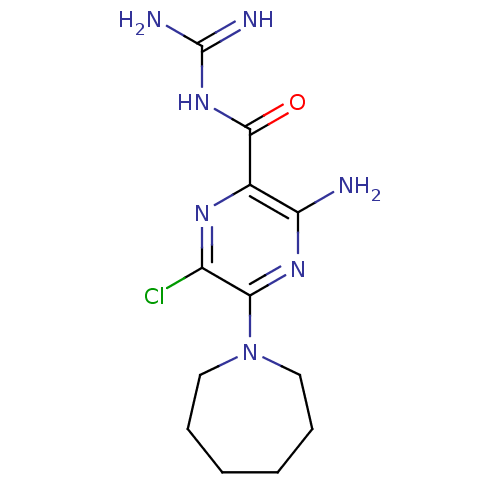
BDBM81818 CAS_1794::CHEMBL1909810::HMA::NSC_1794
SMILES NC(=N)NC(=O)c1nc(Cl)c(nc1N)N1CCCCCC1
InChI Key InChIKey=RQQJJXVETXFINY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 52 hits for monomerid = 81818
Found 52 hits for monomerid = 81818
Affinity DataIC50: 0.190nMAssay Description:Inhibition of human NHE-1More data for this Ligand-Target Pair
TargetCalcium release-activated calcium channel protein 1(Human)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Affinity DataIC50: 171nMAssay Description:Inhibition of rat NHE1 expressed in Chinese hamster ovary cells (AP-1) measured after 9 mins by BCECF-AM staining based NH4Cl pre-pulse methodMore data for this Ligand-Target Pair
Affinity DataIC50: 270nMAssay Description:Displacement of [3H]ZM-241,385 from human N-terminal FLAG-tagged adenosine A2A receptor W246A 6.48 mutant expressed in HEK293 cell membrane by microb...More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Rat)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
TargetAdenosine receptor A1(Bovine)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
TargetDelta-type opioid receptor(Rat)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Affinity DataKi: 1.36E+3nMAssay Description:Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.51E+3nMAssay Description:Inhibition of human NHE5 transfected in chinese hamster PS120 cells measured after 9 mins by BCECF-AM staining based NH4Cl pre-pulse methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of human kidney uPA using chromogenic H-D-Ile-L-Pro-L-Arg-p-nitoraniline as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Rat)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
TargetPotassium voltage-gated channel subfamily H member 2(Human)
University of Wollongong
Curated by ChEMBL
University of Wollongong
Curated by ChEMBL
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibition of recombinant human ERG expressed in CHO cells incubated for 12 mins by whole cell voltage clamp methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibition of NHE1 in human Platelet assessed as reduction in 22Na+ uptakeMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Rat)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
TargetKappa-type opioid receptor(Rat)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M3(Rat)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Affinity DataIC50: 5.10E+3nMAssay Description:Displacement of [3H]ZM-241,385 from human N-terminal FLAG-tagged adenosine A2A receptor expressed in HEK293 cell membrane by microbeta scintillation ...More data for this Ligand-Target Pair
TargetAlpha-2C adrenergic receptor(Rat)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of urokinase-type plasminogen activatorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Pig)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
TargetSodium-dependent serotonin transporter(Rat)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Affinity DataKi: 9.31E+3nMAssay Description:Inhibition of mouse uPA using Z-Gly-Gly-Arg-AMC as substrate after 15 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of plasmin (unknown origin) using chromogenic H-D-Ile-L-Pro-L-Arg-p-nitoraniline as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-1(Rat)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
TargetGamma-aminobutyric acid receptor subunit alpha-1(Rat)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 1B(Rat)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 1A(Rat)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
TargetBeta-1 adrenergic receptor(Rat)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of thrombin (unknown origin) using chromogenic H-D-Ile-L-Pro-L-Arg-p-nitoraniline as substrate by fluorescence assayMore data for this Ligand-Target Pair
Target5-hydroxytryptamine 1D receptor(Bovine)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
TargetVoltage-dependent calcium channel gamma-1 subunit(Human)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
TargetGlycine amidinotransferase, mitochondrial(Rat)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
TargetGastrin/cholecystokinin type B receptor(Rat)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
TargetCysteinyl leukotriene receptor 1(Guinea pig)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 3A(Mouse)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
TargetGastrin/cholecystokinin type B receptor(Rat)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of tPA (unknown origin) using chromogenic H-D-Ile-L-Pro-L-Arg-p-nitoraniline as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetPro-thyrotropin-releasing hormone(Rat)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
TargetAlpha-1A adrenergic receptor(Rat)
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Center For Bio-Pharmaceutical Sciences
Curated by PDSP Ki Database
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of trypsin (unknown origin) using chromogenic H-D-Ile-L-Pro-L-Arg-p-nitoraniline as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of recombinant human Nav1.5alpha expressed in HEK293 cells incubated for 10 mins by voltage clamp methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of activated protein C (unknown origin) using chromogenic L-pyroGlu-L-Pro-L-Arg-p-nitroaniline as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of factor 10a (unknown origin) using chromogenic H-D-Ile-L-Pro-L-Arg-p-nitoraniline as substrate by fluorescence assayMore data for this Ligand-Target Pair