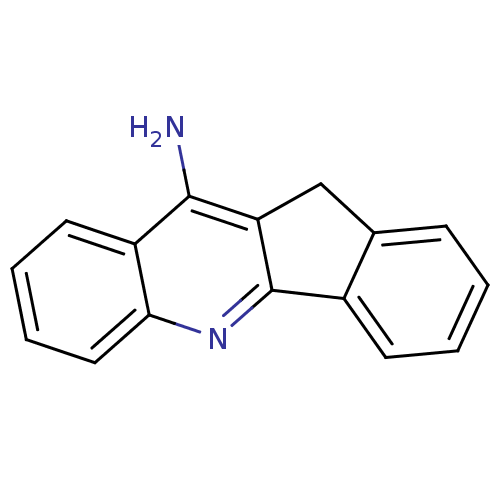
BDBM9074 10H-indeno[1,2-b]quinolin-11-amine::11H-indeno-[1,2-b]-quinolin-10-ylamine deriv. 1a::CHEMBL60167
SMILES Nc1c2Cc3ccccc3-c2nc2ccccc12
InChI Key InChIKey=CMZUAEAPKGOQSL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 9074
Found 10 hits for monomerid = 9074
Affinity DataKi: 210nM IC50: 680nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:In vitro inhibition of acetylcholinesterase, isolated from rat brain.More data for this Ligand-Target Pair
Affinity DataIC50: 3.10E+3nMAssay Description:In vitro inhibition of Butyrylcholinesterase from human plasma.More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
University Of Strathclyde
Curated by ChEMBL
University Of Strathclyde
Curated by ChEMBL
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of neuronal uptake of 5 - Hydroxytryptamine in rat brain homogenateMore data for this Ligand-Target Pair
Affinity DataIC50: 676nMAssay Description:Compound was tested in vitro for inhibitory activity against AcetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 480nMAssay Description:Compound was tested in vitro for inhibitory activity against Acetylcholinesterase in rat cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 4.67E+3nMAssay Description:Compound was tested in vitro for inhibitory activity against ButyrylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+3nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of neuronal uptake of Noradrenaline in rat brain homogenateMore data for this Ligand-Target Pair