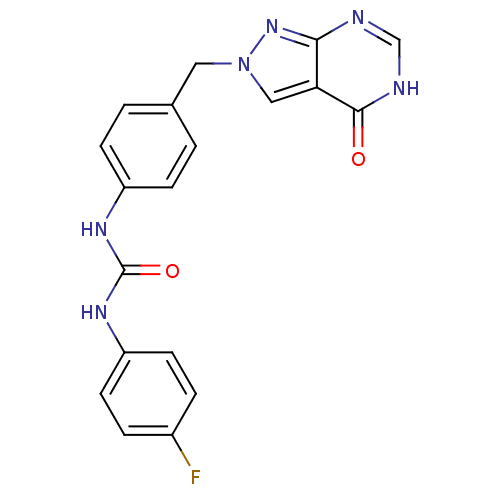
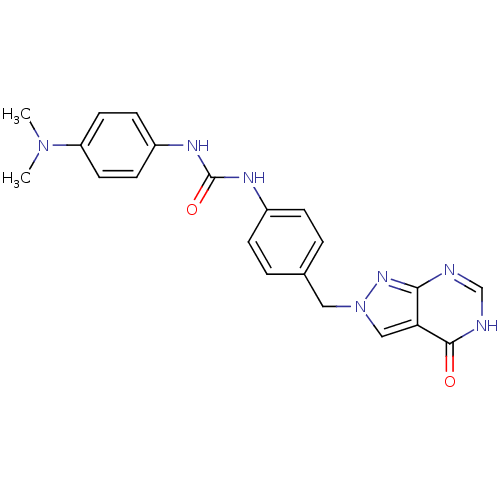
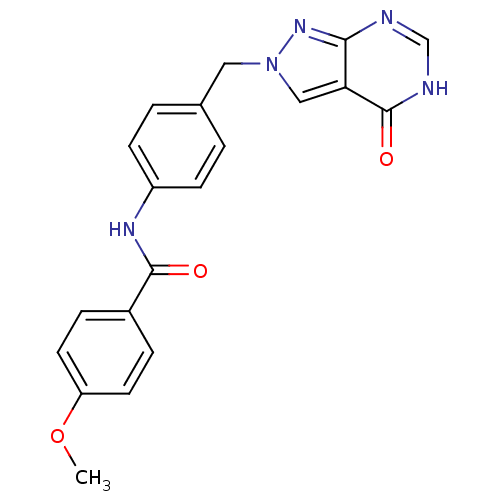
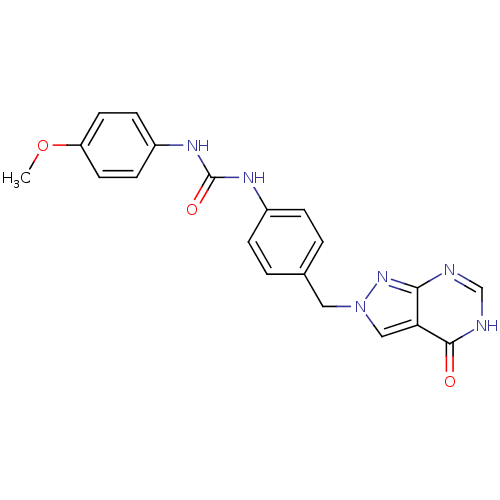
Affinity DataKi: 160nM ΔG°: -39.4kJ/molepH: 7.2 T: 2°CAssay Description:The activity of ADA was determined spectrophotometrically by monitoring the change in absorbance at 262 nm, due to the deamination of adenosine catal...More data for this Ligand-Target Pair
Affinity DataKi: 300nMAssay Description:Inhibition of human APN expressed in HEK293 cells preincubated for 20 mins followed by L-leucine-7-amido-4-methylcoumarin addition by fluorescence as...More data for this Ligand-Target Pair
Affinity DataKi: 510nM ΔG°: -36.5kJ/molepH: 7.2 T: 2°CAssay Description:The activity of ADA was determined spectrophotometrically by monitoring the change in absorbance at 262 nm, due to the deamination of adenosine catal...More data for this Ligand-Target Pair
Affinity DataKi: 960nM ΔG°: -34.9kJ/molepH: 7.2 T: 2°CAssay Description:The activity of ADA was determined spectrophotometrically by monitoring the change in absorbance at 262 nm, due to the deamination of adenosine catal...More data for this Ligand-Target Pair
Affinity DataKi: 1.14E+3nM ΔG°: -34.5kJ/molepH: 7.2 T: 2°CAssay Description:The activity of ADA was determined spectrophotometrically by monitoring the change in absorbance at 262 nm, due to the deamination of adenosine catal...More data for this Ligand-Target Pair
Affinity DataKi: 1.15E+3nM ΔG°: -34.5kJ/molepH: 7.2 T: 2°CAssay Description:The activity of ADA was determined spectrophotometrically by monitoring the change in absorbance at 262 nm, due to the deamination of adenosine catal...More data for this Ligand-Target Pair
Affinity DataKi: 2.01E+3nM ΔG°: -33.1kJ/molepH: 7.2 T: 2°CAssay Description:The activity of ADA was determined spectrophotometrically by monitoring the change in absorbance at 262 nm, due to the deamination of adenosine catal...More data for this Ligand-Target Pair
Affinity DataKi: 2.42E+3nM ΔG°: -32.6kJ/molepH: 7.2 T: 2°CAssay Description:The activity of ADA was determined spectrophotometrically by monitoring the change in absorbance at 262 nm, due to the deamination of adenosine catal...More data for this Ligand-Target Pair
Affinity DataKi: 1.27E+4nM ΔG°: -28.4kJ/molepH: 7.2 T: 2°CAssay Description:The activity of ADA was determined spectrophotometrically by monitoring the change in absorbance at 262 nm, due to the deamination of adenosine catal...More data for this Ligand-Target Pair
Affinity DataKi: 1.56E+4nM ΔG°: -27.9kJ/molepH: 7.2 T: 2°CAssay Description:The activity of ADA was determined spectrophotometrically by monitoring the change in absorbance at 262 nm, due to the deamination of adenosine catal...More data for this Ligand-Target Pair
Affinity DataKi: 2.83E+4nM ΔG°: -26.4kJ/molepH: 7.2 T: 2°CAssay Description:The activity of ADA was determined spectrophotometrically by monitoring the change in absorbance at 262 nm, due to the deamination of adenosine catal...More data for this Ligand-Target Pair
Affinity DataKi: 4.79E+4nM ΔG°: -25.1kJ/molepH: 7.2 T: 2°CAssay Description:The activity of ADA was determined spectrophotometrically by monitoring the change in absorbance at 262 nm, due to the deamination of adenosine catal...More data for this Ligand-Target Pair
Affinity DataKi: 8.57E+4nM ΔG°: -23.6kJ/molepH: 7.2 T: 2°CAssay Description:The activity of ADA was determined spectrophotometrically by monitoring the change in absorbance at 262 nm, due to the deamination of adenosine catal...More data for this Ligand-Target Pair
Affinity DataKi: 1.08E+5nM ΔG°: -23.0kJ/molepH: 7.2 T: 2°CAssay Description:The activity of ADA was determined spectrophotometrically by monitoring the change in absorbance at 262 nm, due to the deamination of adenosine catal...More data for this Ligand-Target Pair
Affinity DataKi: 1.86E+5nM ΔG°: -21.6kJ/molepH: 7.2 T: 2°CAssay Description:The activity of ADA was determined spectrophotometrically by monitoring the change in absorbance at 262 nm, due to the deamination of adenosine catal...More data for this Ligand-Target Pair
Affinity DataIC50: 0.190nMAssay Description:Antagonist activity at ERalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.260nMAssay Description:Antagonist activity at ERalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.330nMAssay Description:Antagonist activity at ERalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.390nMAssay Description:Antagonist activity at ERalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMAssay Description:Inhibition of MMP2 (unknown origin) preincubated for 1 hr followed by (QF)-24 substrate addition measured at 1 min time interval for 1 hr by fluoresc...More data for this Ligand-Target Pair
Affinity DataIC50: 0.670nMAssay Description:Antagonist activity at ERalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.710nMAssay Description:Antagonist activity at ERalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.740nMAssay Description:Antagonist activity at ERalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.75nMAssay Description:Antagonist activity at ERalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.760nMAssay Description:Antagonist activity at ERalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.810nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 0.890nMAssay Description:Antagonist activity at ERbeta (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.890nMAssay Description:Antagonist activity at ERalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Antagonist activity at ERalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Antagonist activity at ERalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Antagonist activity at ERalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.41nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 1.42nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 1.79nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 1.84nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 1.86nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 2.09nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 2.46nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 2.57nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 2.67nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 2.92nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk2(Homo sapiens (Human))
The Institute Of Cancer Research
Curated by ChEMBL
The Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of CHK2 by DELFIA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.01nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Inhibition of MMP9 (unknown origin) preincubated for 1 hr followed by (QF)-24 substrate addition measured at 1 min time interval for 1 hr by fluoresc...More data for this Ligand-Target Pair
Affinity DataIC50: 3.31nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 3.58nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:Antagonist activity at ERbeta (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.72nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 3.72nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 3.91nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)