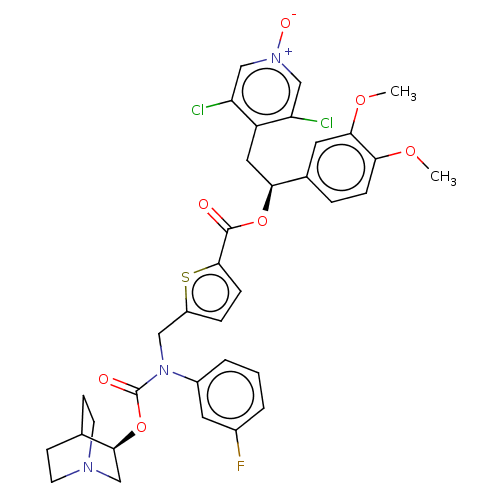
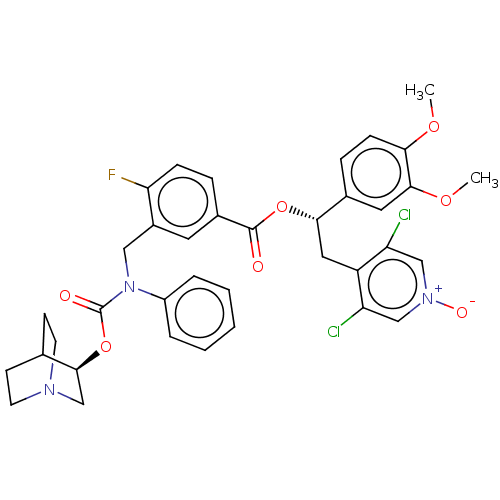
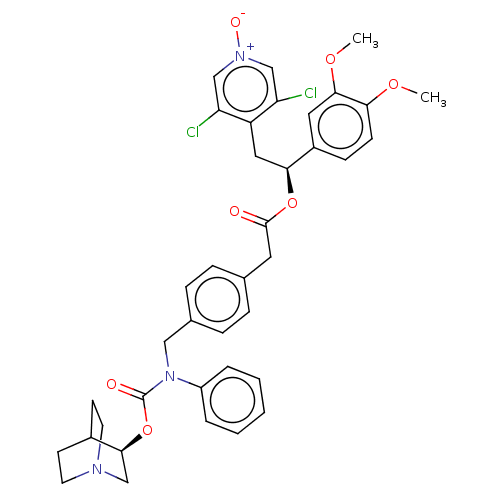
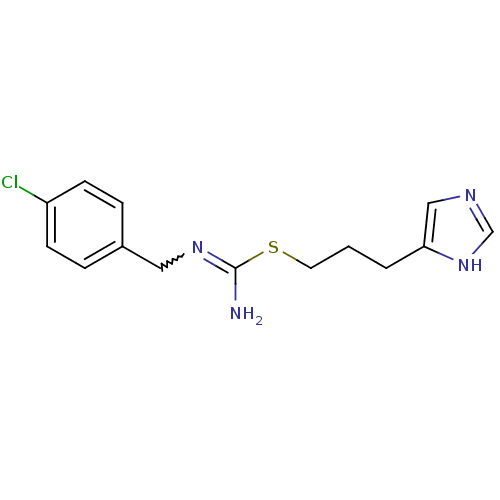
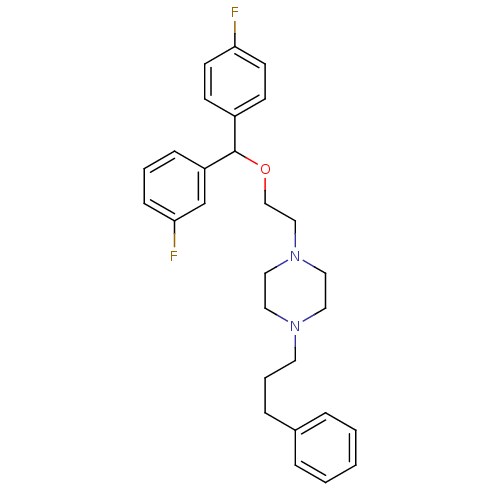
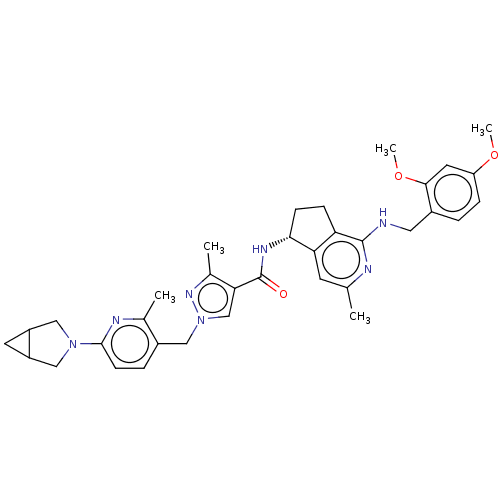
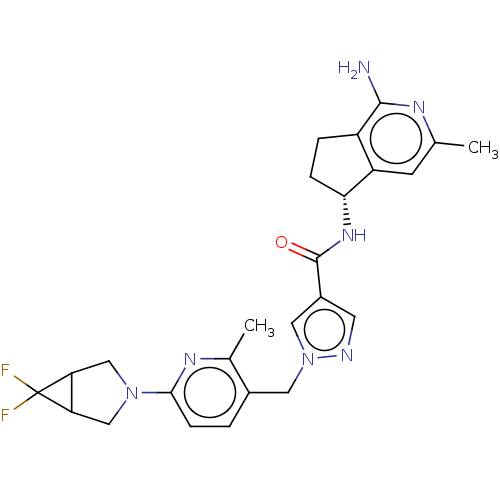
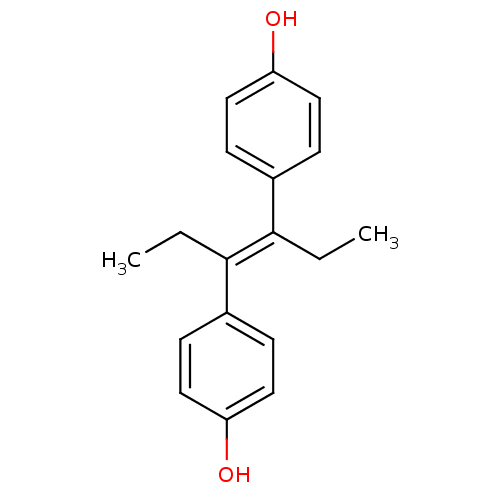
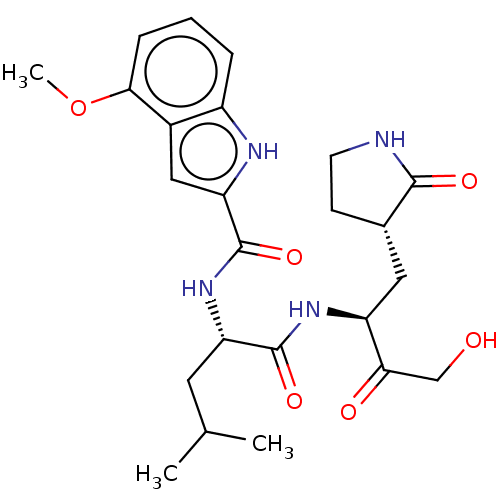
Affinity DataKi: 0.0330nMAssay Description:PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc...More data for this Ligand-Target Pair
Affinity DataKi: 0.0340nMAssay Description:PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc...More data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
University Of North Carolina At Chapel Hill
Curated by PDSP Ki Database
University Of North Carolina At Chapel Hill
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
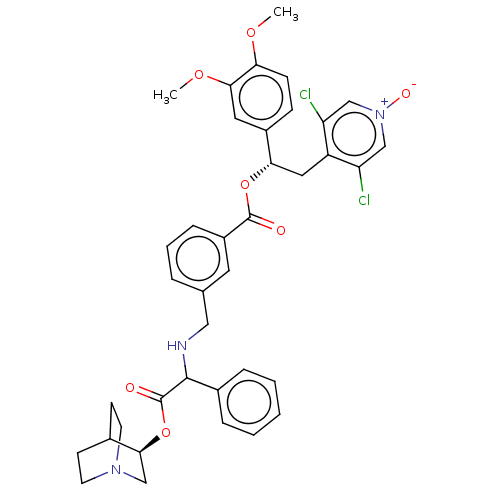
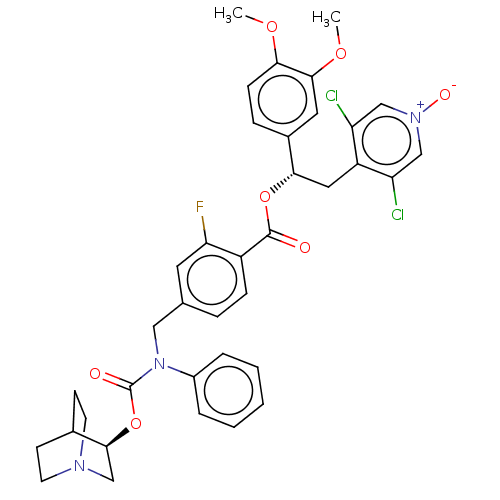
Affinity DataKi: 0.100nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.120nMAssay Description:PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc...More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.126nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.126nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.126nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.126nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.158nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.158nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M2 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.158nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.158nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetHistamine H1 receptor(Cavia porcellus (domestic guinea pig))
University Of North Carolina At Chapel Hill
Curated by PDSP Ki Database
University Of North Carolina At Chapel Hill
Curated by PDSP Ki Database
Affinity DataKi: 0.160nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor betaMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M2 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.25nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alphaMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.251nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.270nMAssay Description:PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc...More data for this Ligand-Target Pair
TargetHistamine H1 receptor(Cavia porcellus (domestic guinea pig))
University Of North Carolina At Chapel Hill
Curated by PDSP Ki Database
University Of North Carolina At Chapel Hill
Curated by PDSP Ki Database
Affinity DataKi: 0.300nMAssay Description:PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295...More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.316nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.316nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.316nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.316nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.316nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
University Of North Carolina At Chapel Hill
Curated by PDSP Ki Database
University Of North Carolina At Chapel Hill
Curated by PDSP Ki Database
Affinity DataKi: 0.339nM ΔG°: -54.1kJ/molepH: 7.4 T: 2°CAssay Description:Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count...More data for this Ligand-Target Pair
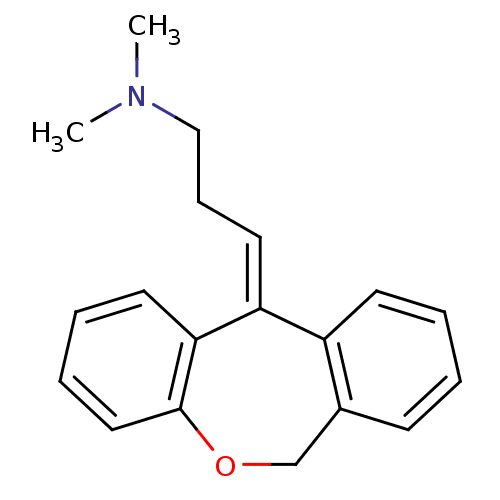
Affinity DataKi: 0.360nMAssay Description:Competitive binding versus [N-methyl-3H]-WIN 35,428 in murine kidney cells transfected with human dopamine transporterMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.398nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.398nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.398nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295...More data for this Ligand-Target Pair
Affinity DataKi: 0.407nM ΔG°: -53.6kJ/molepH: 7.4 T: 2°CAssay Description:Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count...More data for this Ligand-Target Pair
Affinity DataKi: 0.480nMAssay Description:In vitro affinity determined using [3H]-WIN- 35428 in murine kidney cells transfected with human dopamine transporter (DAT)More data for this Ligand-Target Pair
Affinity DataKi: 0.490nMAssay Description:In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Human KLKB1 (1.78 nM or 0.025 U/mL; Enzyme Research Laboratories) was incubated at 24° C. with 0.25 mM fluorogenic substrate H-Pro-Phe-Arg-AMC (11295...More data for this Ligand-Target Pair






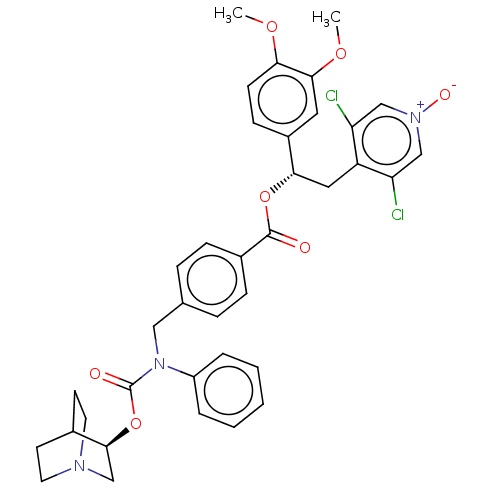
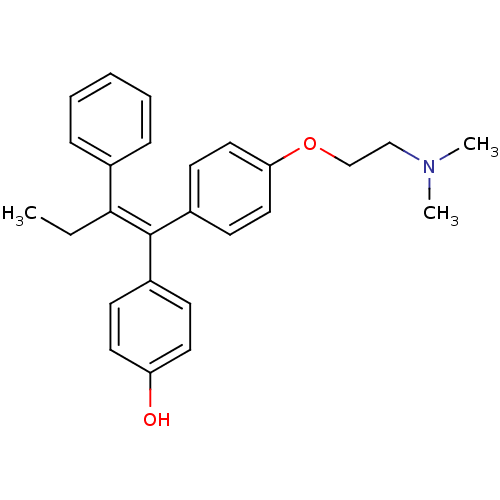
 3D Structure (crystal)
3D Structure (crystal)