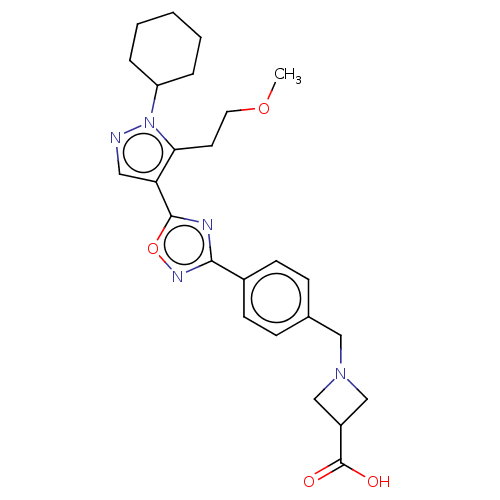
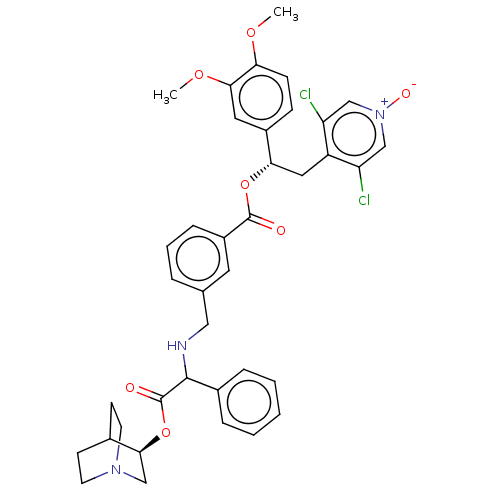
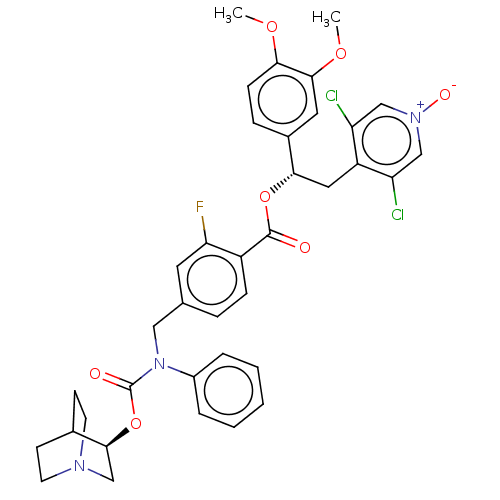
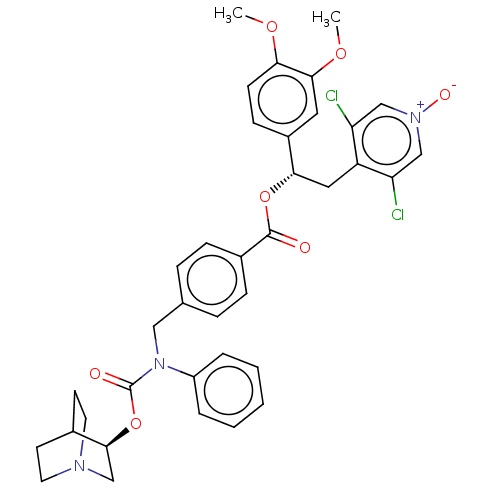
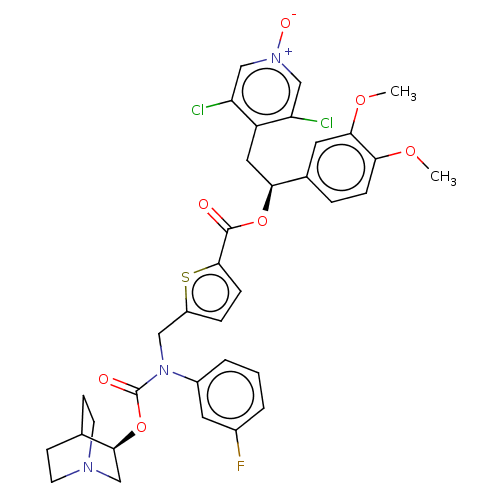
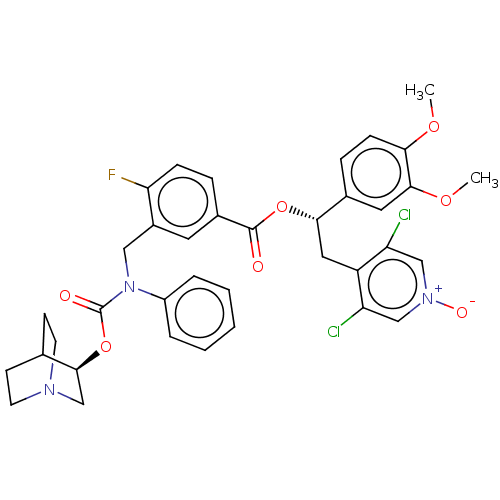
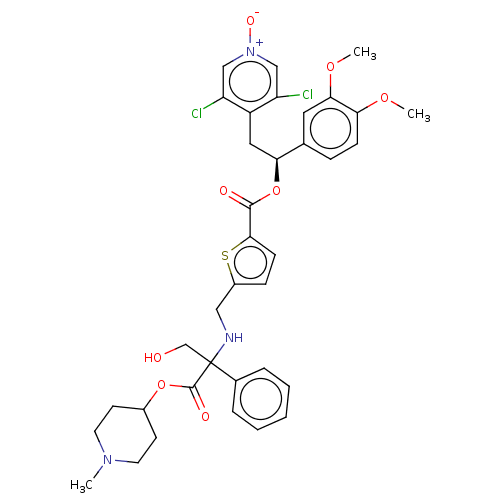
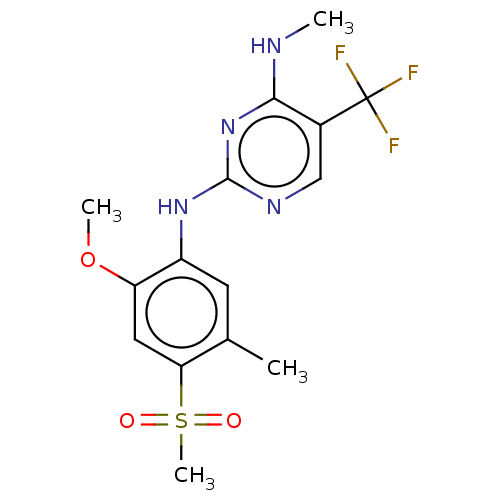
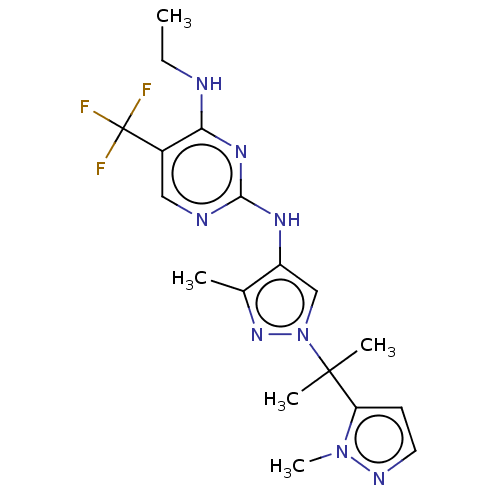
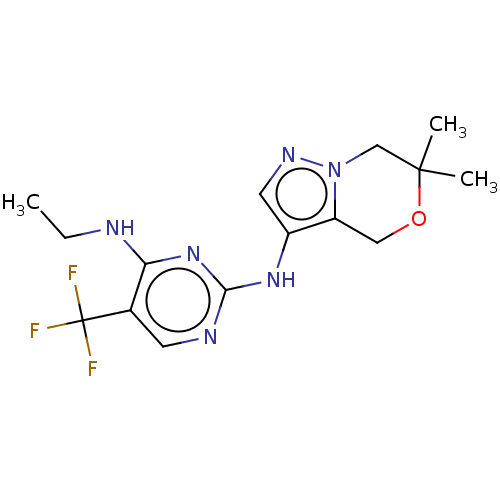
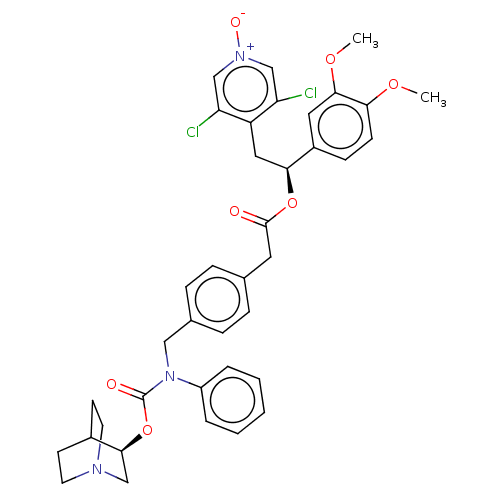
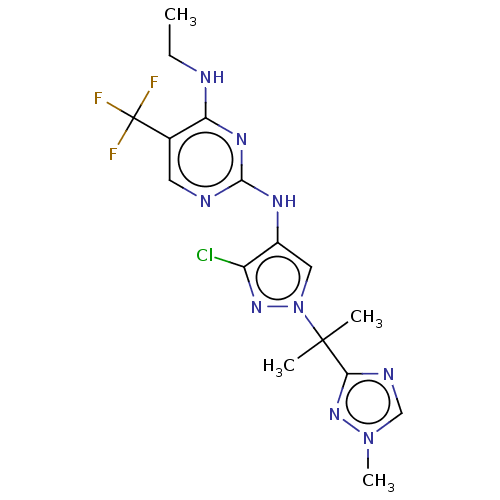
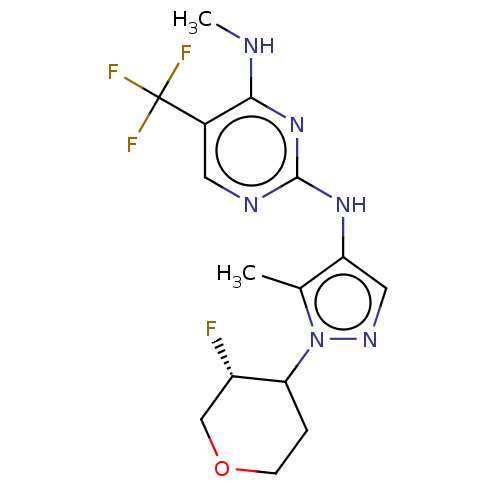
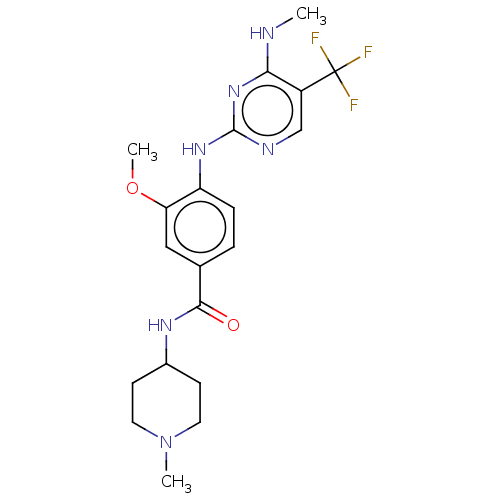
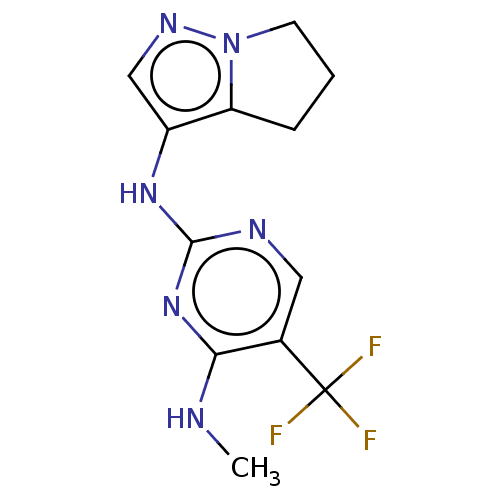
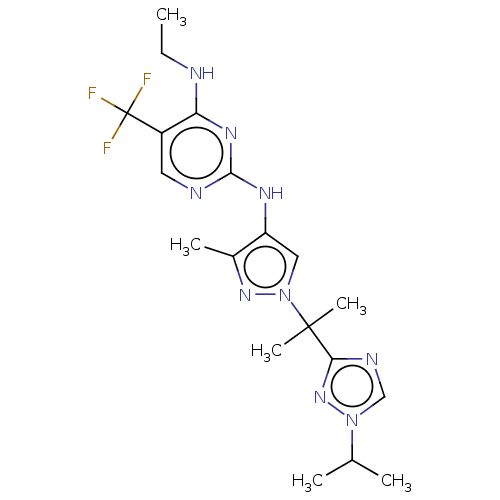
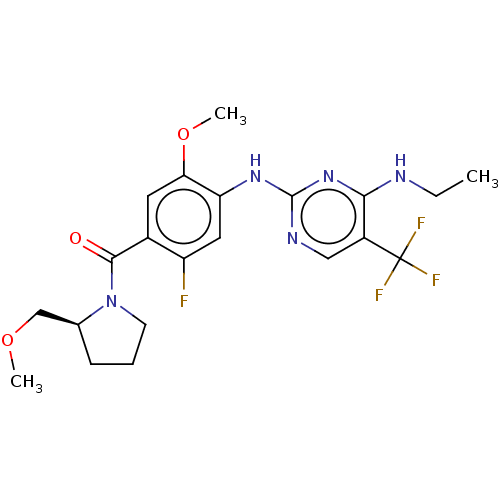
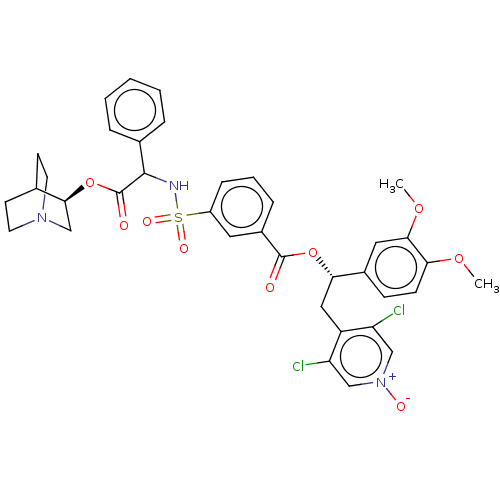
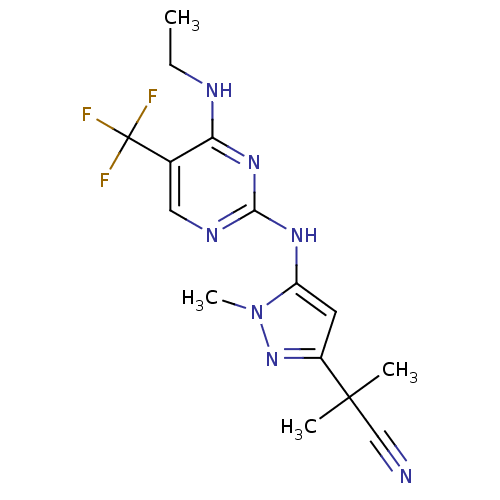
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.100nM ΔG°: -53.1kJ/molepH: 7.4 T: 2°CAssay Description:Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 355-GTPgammaS binding studies. Cells wer...More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.126nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.126nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.126nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.126nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.158nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.158nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M2 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.158nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.158nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M2 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.230nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.251nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38...More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3...More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.316nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.316nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.316nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.316nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.316nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.398nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.398nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.398nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3...More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.501nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.501nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.501nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.540nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.590nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nM ΔG°: -48.9kJ/molepH: 7.4 T: 2°CAssay Description:Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 355-GTPgammaS binding studies. Cells wer...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ...More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.631nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.631nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.631nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.631nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nM ΔG°: -48.6kJ/molepH: 7.4 T: 2°CAssay Description:Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 355-GTPgammaS binding studies. Cells wer...More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Chiesi Farmaceutici
Curated by ChEMBL
Chiesi Farmaceutici
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3...More data for this Ligand-Target Pair