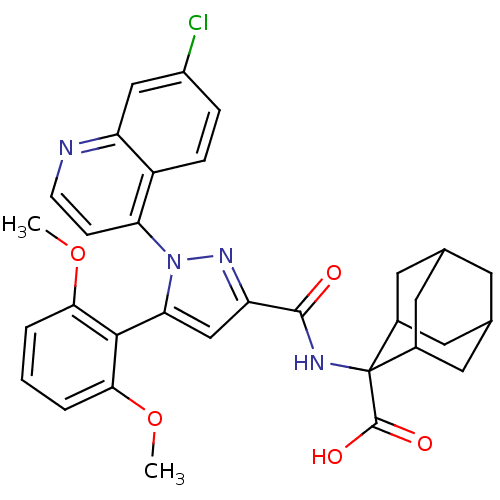
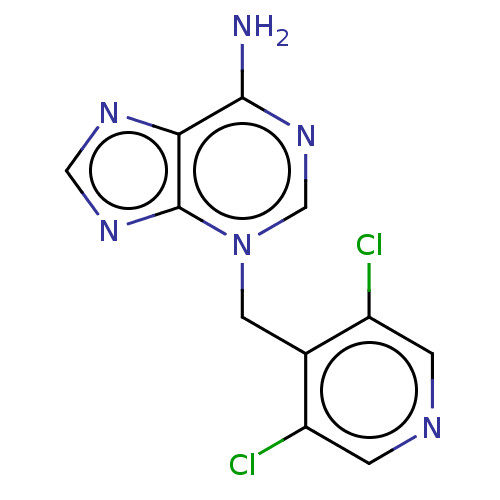
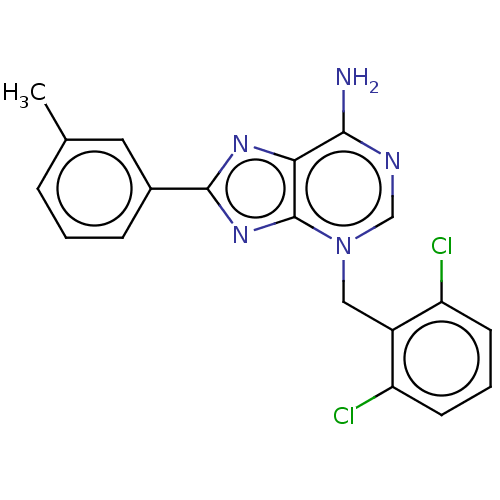
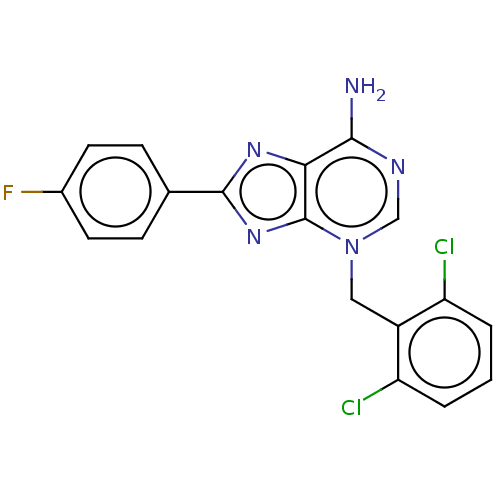
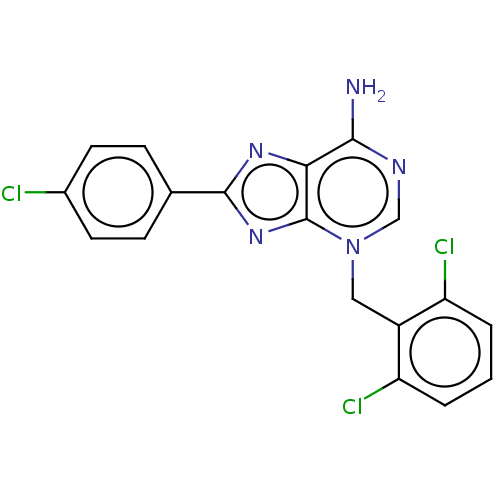
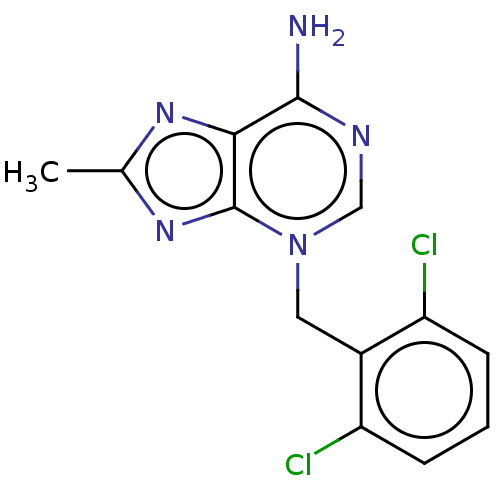
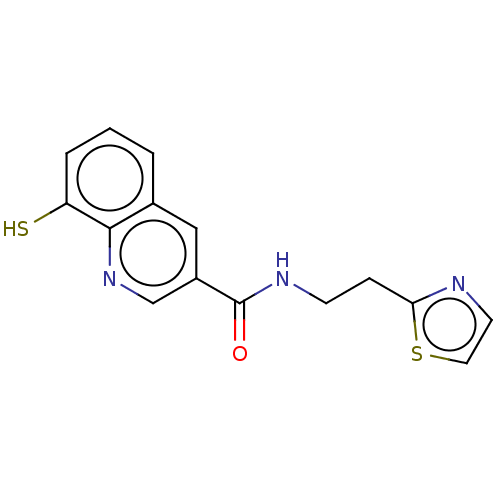
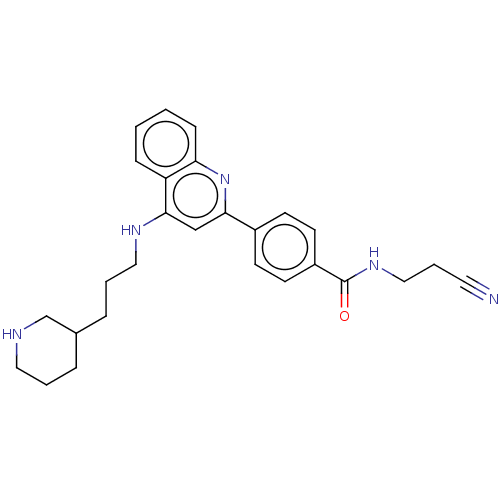
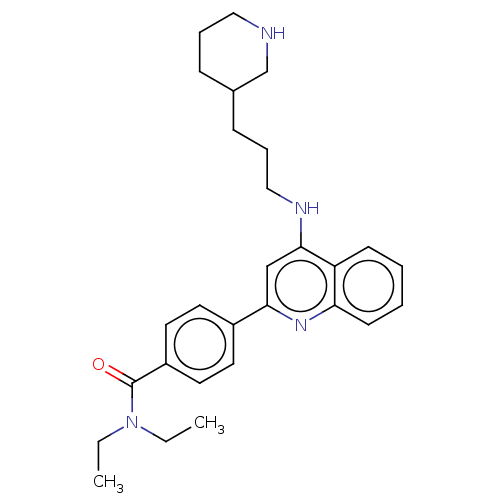
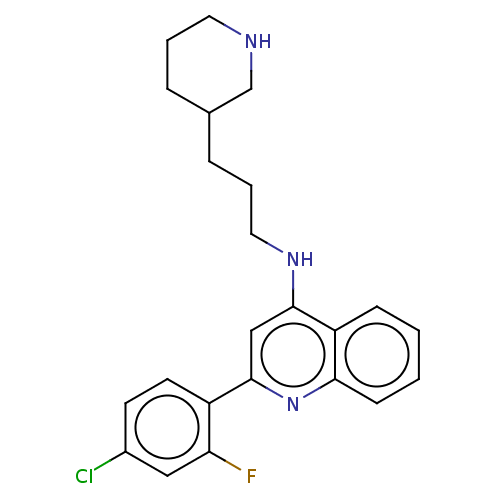
TargetSigma non-opioid intracellular receptor 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 410nMAssay Description:Binding affinity to sigma-1 receptor (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 7(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 690nMAssay Description:Competitive inhibition of recombinant HePTP expressed in Escherichia coli by Michaelis-Menten kinetic analysisMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 3A(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Binding affinity to 5-HT3 receptor (unknown origin)More data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.42E+3nMAssay Description:Binding affinity to histamine H1 receptor (unknown origin)More data for this Ligand-Target Pair
TargetTranslocator protein(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 2.54E+3nMAssay Description:Binding affinity to PBR receptor (unknown origin)More data for this Ligand-Target Pair
TargetIntestinal-type alkaline phosphatase(Mus musculus)
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 3.20E+3nMAssay Description:Competitive inhibition of mouse duodenal-specific FLAG-tagged IAP expressed in African green monkey COS1 cells using p-nitrophenyl phosphate as subst...More data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 3.30E+3nMAssay Description:Inhibition of NTS1 receptor (unknown origin)More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 3.40E+3nMAssay Description:Inhibition of MOR (unknown origin)More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 5.20E+3nMAssay Description:Inhibition of DOR (unknown origin)More data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.00E+4nMAssay Description:Inhibition of NTS1 receptor (unknown origin)More data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of DAT (unknown origin)More data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of DAT (unknown origin)More data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.240nMAssay Description:Displacement of [125I]-neurotensin from NTR1 (unknown origin)More data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.240nMAssay Description:Displacement of [125I]-neurotensin from NTR1 in HUVEC after 1 hr by gamma counting analysisMore data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Antagonist activity at NTR1 (unknown origin)More data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human LMPTP-A using OMFP or pNPP as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of recombinant human LMPTP-A using OMFP or pNPP as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Antagonist activity at NTR1 (unknown origin) expressed in human U2OS cells coexpressing beta-arrestin assessed as inhibition of ML314-induced effect ...More data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Rattus norvegicus)
Sanford Burnham Prebys Medical Discovery Institute
Curated by ChEMBL
Sanford Burnham Prebys Medical Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 82nMAssay Description:Displacement of [125I]neurotensin from rat brain NTR1More data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 83nMAssay Description:Inhibition of recombinant human LMPTP-A using OMFP or pNPP as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 87nMAssay Description:Inhibition of recombinant human LMPTP-A using OMFP or pNPP as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 104nMAssay Description:Inhibition of recombinant human LMPTP-A using OMFP or pNPP as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 106nMAssay Description:Inhibition of recombinant human LMPTP-A using OMFP or pNPP as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 117nMAssay Description:Inhibition of recombinant human LMPTP-A using OMFP or pNPP as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 119nMAssay Description:Inhibition of recombinant human LMPTP-A using OMFP or pNPP as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 147nMAssay Description:Inhibition of recombinant human LMPTP-A using OMFP or pNPP as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 171nMAssay Description:Inhibition of recombinant human LMPTP-A using OMFP or pNPP as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 239nMAssay Description:Inhibition of recombinant human LMPTP-A using OMFP or pNPP as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 239nMAssay Description:Inhibition of recombinant human LMPTP-A using OMFP or pNPP as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 245nMAssay Description:Inhibition of recombinant human LMPTP-A using OMFP or pNPP as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 288nMAssay Description:Inhibition of recombinant human LMPTP-A using OMFP or pNPP as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 306nMAssay Description:Inhibition of recombinant human LMPTP-A using OMFP or pNPP as substrate by fluorescence assayMore data for this Ligand-Target Pair
Target26S proteasome non-ATPase regulatory subunit 14(Homo sapiens (Human))
California Institute Of Technology
California Institute Of Technology
Affinity DataIC50: 340nMpH: 7.5 T: 2°CAssay Description:Fluorescence polarization assays were performed in low-volume 384-well solid black plates (Molecular Devices) in quadruplicate. The assays were perfo...More data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 380nMAssay Description:Inhibition of recombinant human LMPTP-A using OMFP or pNPP as substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetIntestinal-type alkaline phosphatase(Mus musculus)
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataIC50: 400nMAssay Description:Inhibition of mouse duodenal-specific FLAG-tagged IAP expressed in African green monkey COS1 cells after 30 mins by chemiluminescence assayMore data for this Ligand-Target Pair
TargetPotassium channel subfamily K member 2(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: 500nMAssay Description:1. Prepare Reagents as described in section F. Recipe.2. Using LabCyte Echo, transfer 60 nL from 2 mM test compound source plate into assay plate Col...More data for this Ligand-Target Pair
TargetPotassium channel subfamily K member 2(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: 500nMAssay Description:1. Prepare Reagents as described in section F. Recipe.2. Using LabCyte Echo, transfer 60 nL from 2 mM test compound source plate into assay plate Col...More data for this Ligand-Target Pair
TargetPotassium channel subfamily K member 2(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: 500nMAssay Description:1. Prepare Reagents as described in section F. Recipe.2. Using LabCyte Echo, transfer 60 nL from 2 mM test compound source plate into assay plate Col...More data for this Ligand-Target Pair
TargetPotassium channel subfamily K member 2(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: 500nMAssay Description:1. Prepare Reagents as described in section F. Recipe.2. Using LabCyte Echo, transfer 60 nL from 2 mM test compound source plate into assay plate Col...More data for this Ligand-Target Pair
TargetPotassium channel subfamily K member 2(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: 500nMAssay Description:1. Prepare Reagents as described in section F. Recipe.2. Using LabCyte Echo, transfer 60 nL from 2 mM test compound source plate into assay plate Col...More data for this Ligand-Target Pair
TargetPotassium channel subfamily K member 2(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: 500nMAssay Description:1. Prepare Reagents as described in section F. Recipe.2. Using LabCyte Echo, transfer 60 nL from 2 mM test compound source plate into assay plate Col...More data for this Ligand-Target Pair
TargetPotassium channel subfamily K member 2(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: 500nMAssay Description:1. Prepare Reagents as described in section F. Recipe.2. Using LabCyte Echo, transfer 60 nL from 2 mM test compound source plate into assay plate Col...More data for this Ligand-Target Pair
TargetPotassium channel subfamily K member 2(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: 500nMAssay Description:1. Prepare Reagents as described in section F. Recipe.2. Using LabCyte Echo, transfer 60 nL from 2 mM test compound source plate into assay plate Col...More data for this Ligand-Target Pair
TargetPotassium channel subfamily K member 2(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: 500nMAssay Description:1. Prepare Reagents as described in section F. Recipe.2. Using LabCyte Echo, transfer 60 nL from 2 mM test compound source plate into assay plate Col...More data for this Ligand-Target Pair
TargetPotassium channel subfamily K member 2(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: 500nMAssay Description:1. Prepare Reagents as described in section F. Recipe.2. Using LabCyte Echo, transfer 60 nL from 2 mM test compound source plate into assay plate Col...More data for this Ligand-Target Pair
TargetPotassium channel subfamily K member 2(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: 500nMAssay Description:1. Prepare Reagents as described in section F. Recipe.2. Using LabCyte Echo, transfer 60 nL from 2 mM test compound source plate into assay plate Col...More data for this Ligand-Target Pair
TargetPotassium channel subfamily K member 2(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: 500nMAssay Description:1. Prepare Reagents as described in section F. Recipe.2. Using LabCyte Echo, transfer 60 nL from 2 mM test compound source plate into assay plate Col...More data for this Ligand-Target Pair
TargetPotassium channel subfamily K member 2(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: 500nMAssay Description:1. Prepare Reagents as described in section F. Recipe.2. Using LabCyte Echo, transfer 60 nL from 2 mM test compound source plate into assay plate Col...More data for this Ligand-Target Pair
TargetPotassium channel subfamily K member 2(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: 500nMAssay Description:1. Prepare Reagents as described in section F. Recipe.2. Using LabCyte Echo, transfer 60 nL from 2 mM test compound source plate into assay plate Col...More data for this Ligand-Target Pair
TargetPotassium channel subfamily K member 2(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: 500nMAssay Description:1. Prepare Reagents as described in section F. Recipe.2. Using LabCyte Echo, transfer 60 nL from 2 mM test compound source plate into assay plate Col...More data for this Ligand-Target Pair


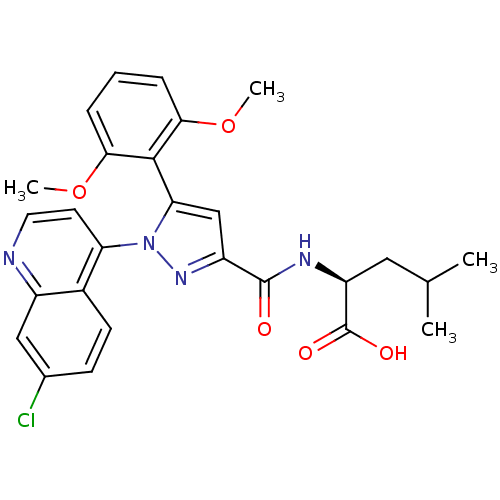
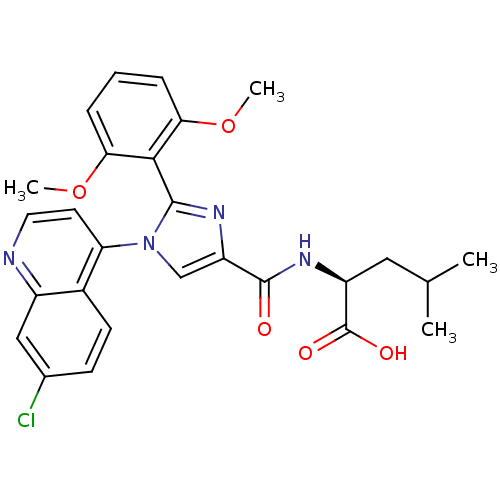
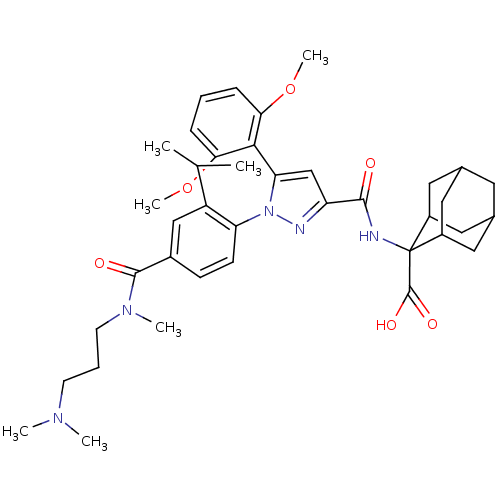
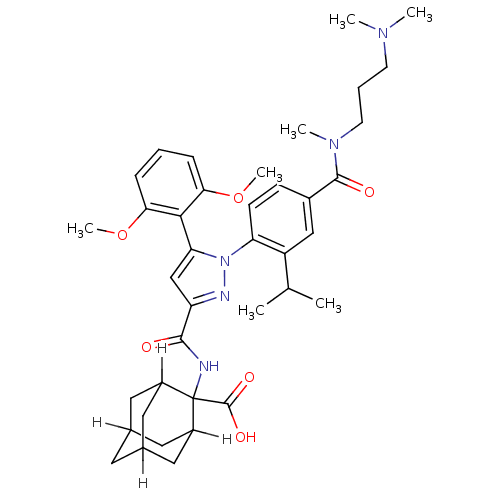

 3D Structure (crystal)
3D Structure (crystal)