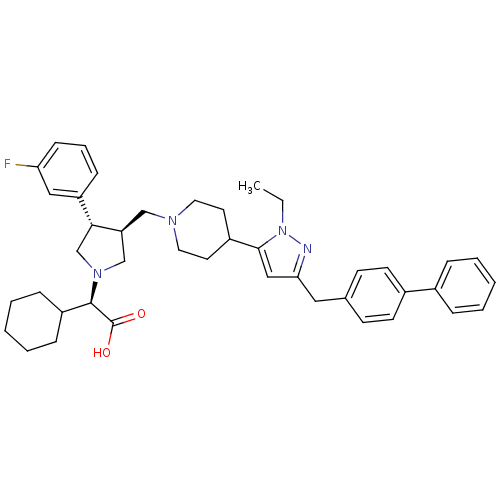
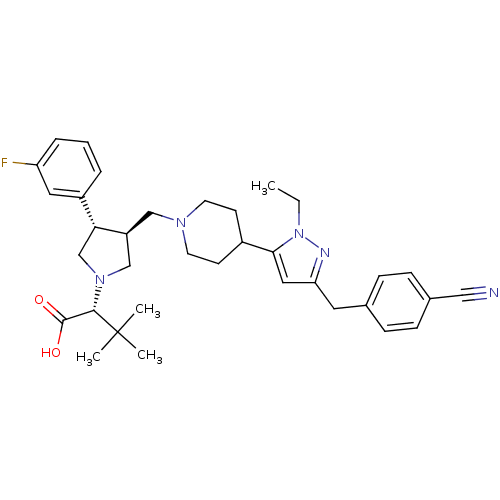
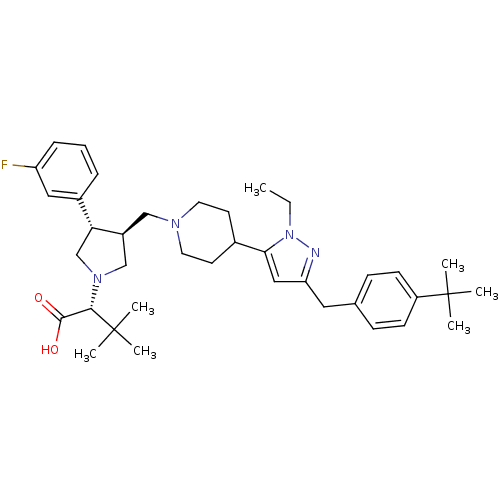
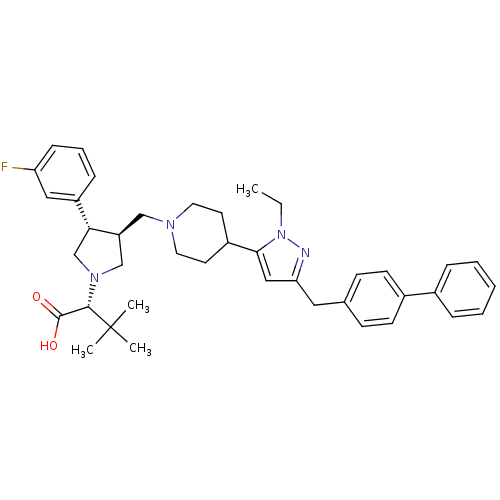
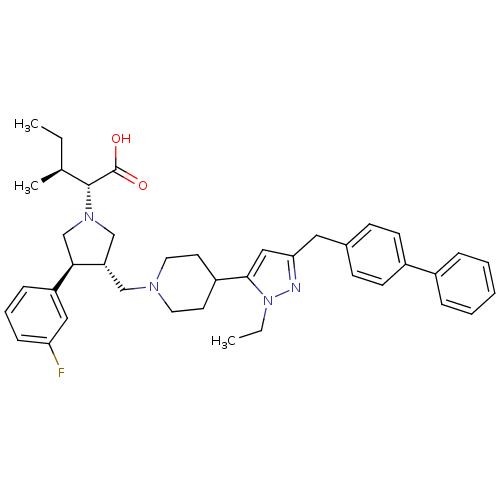
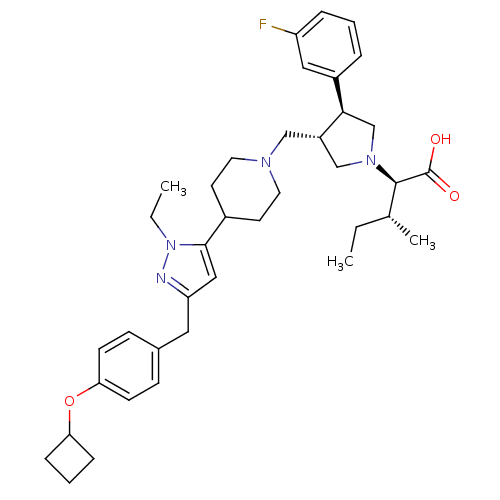
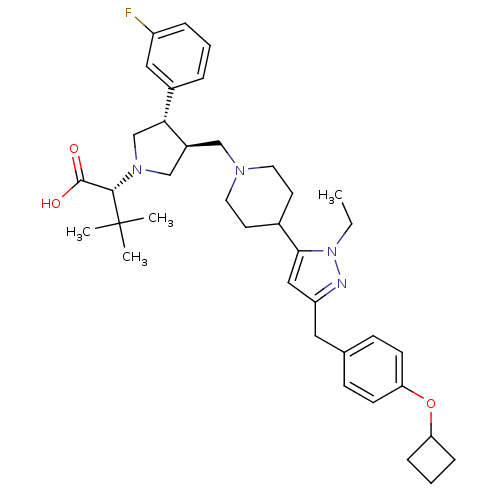
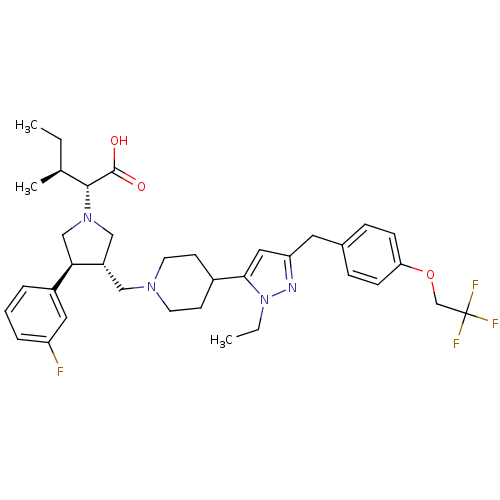
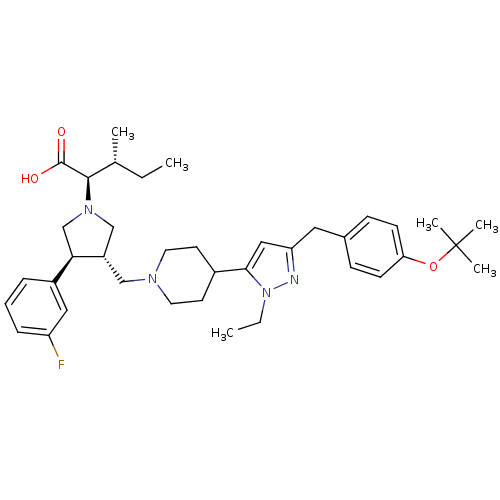
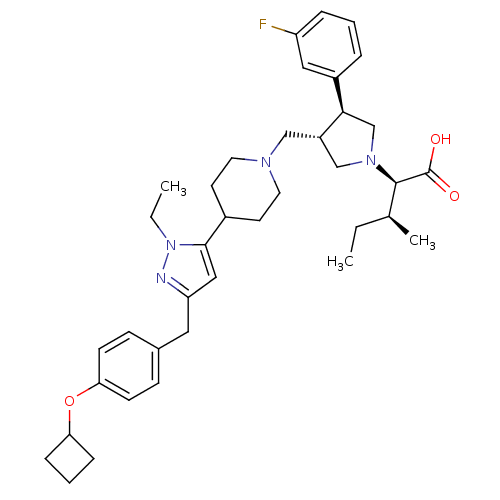
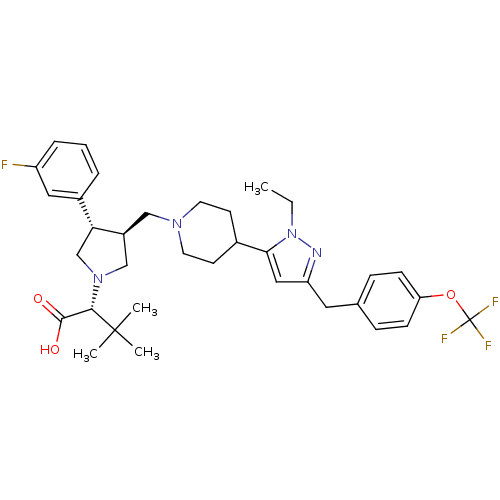
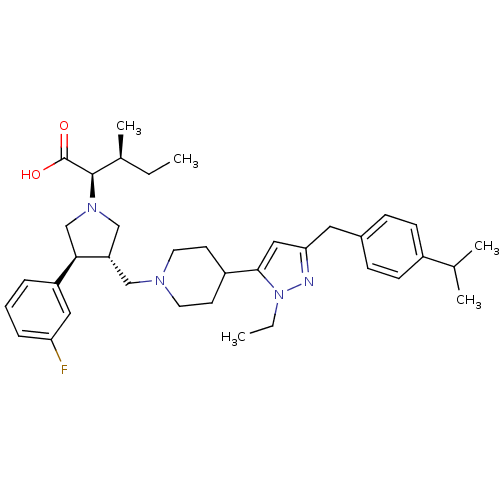
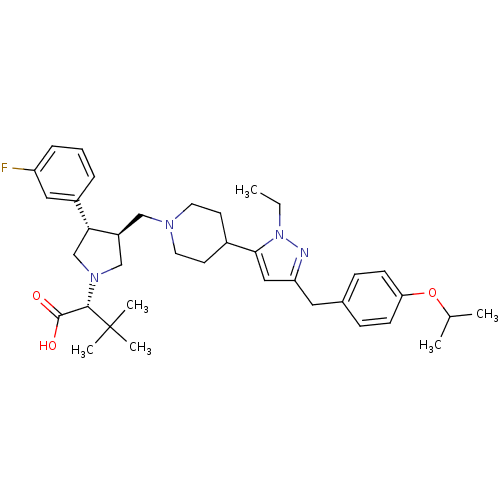
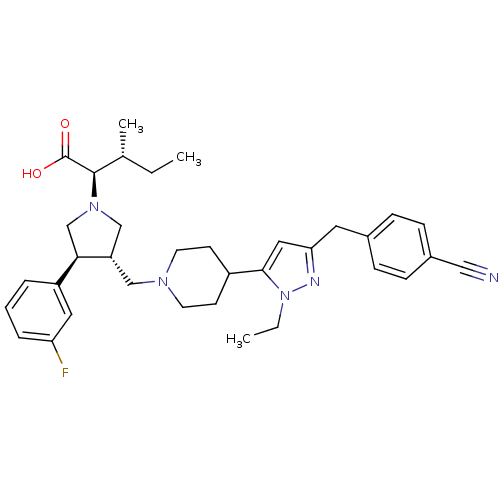
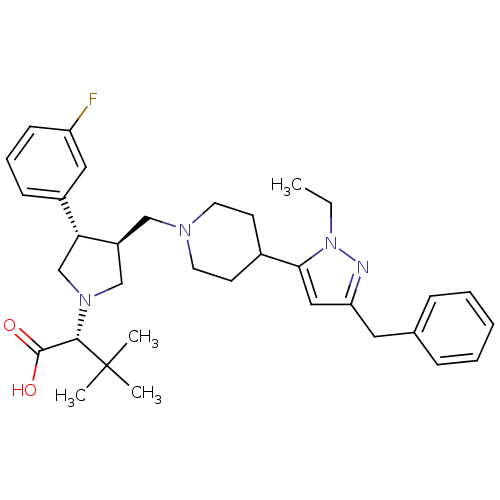
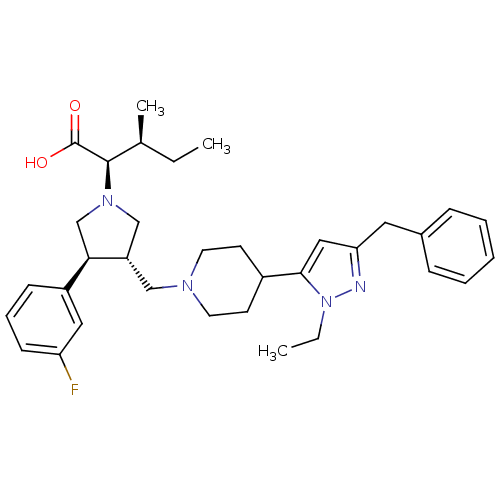
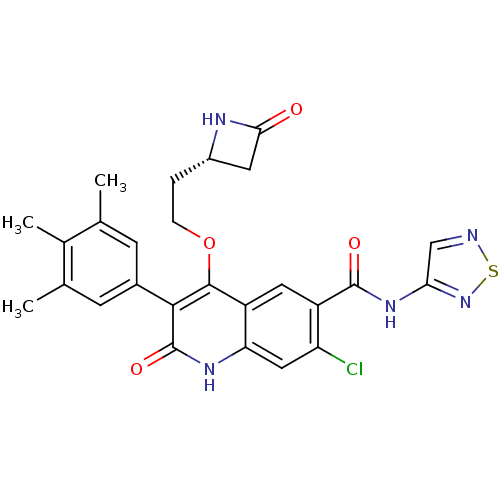
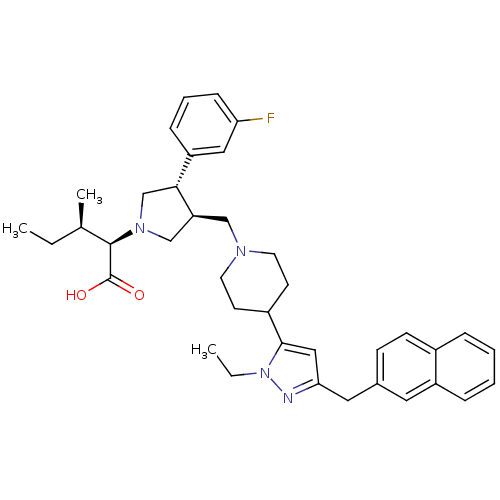
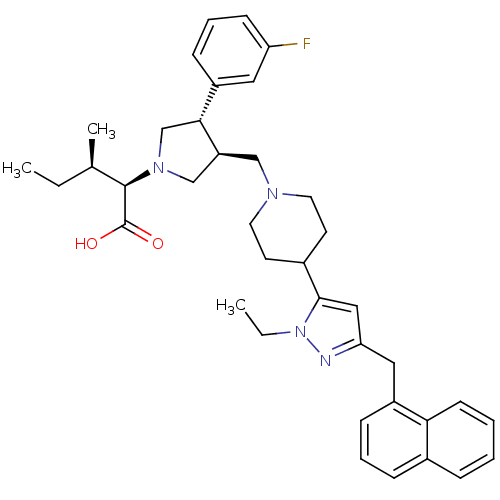
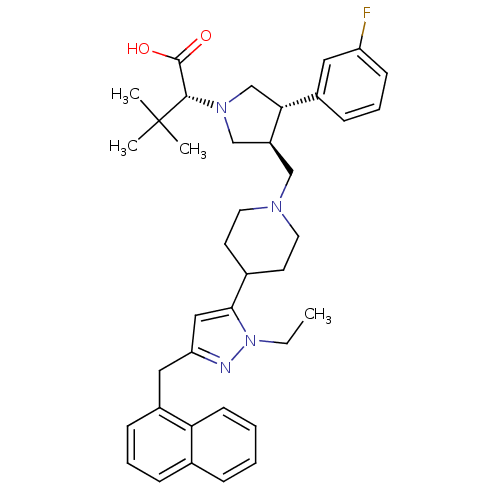
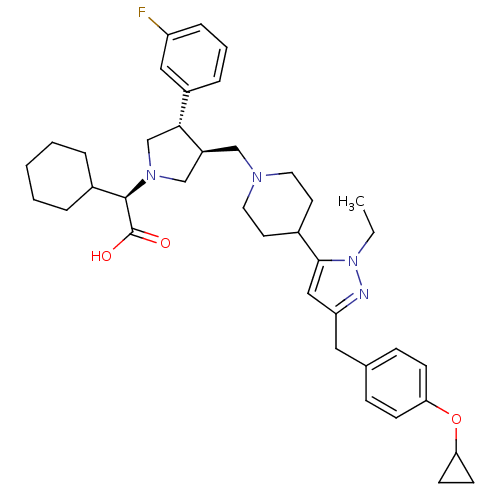
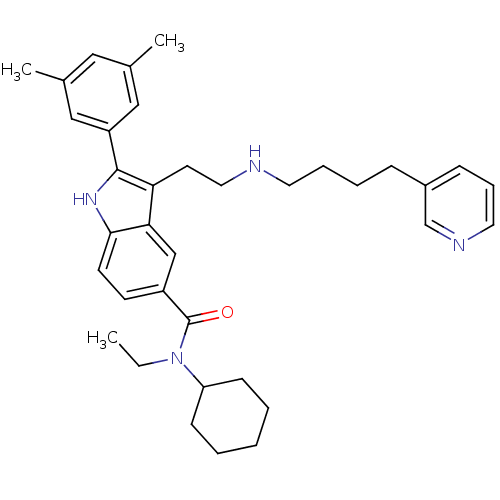
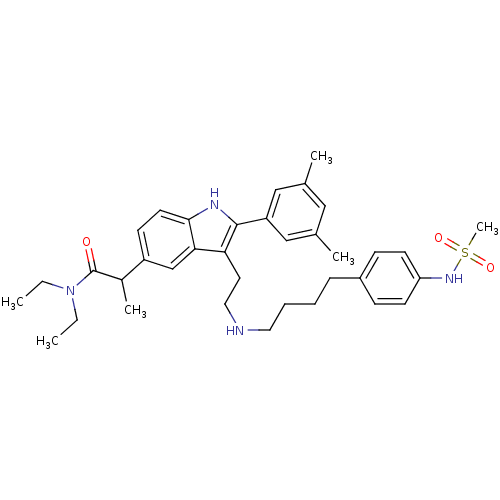
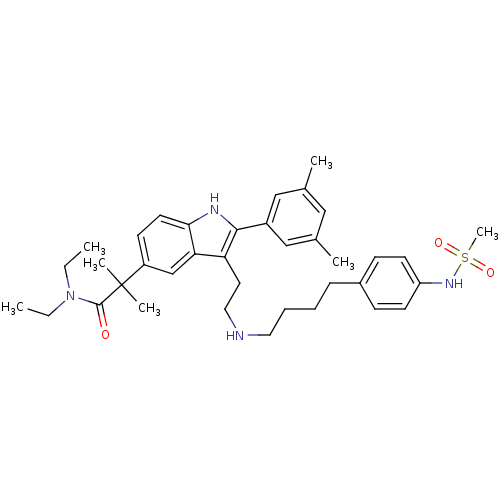
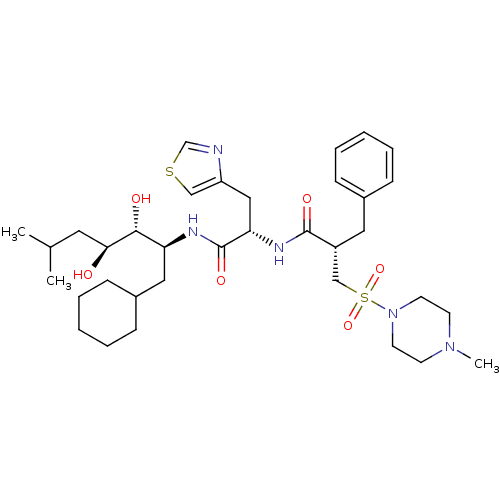
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 400nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
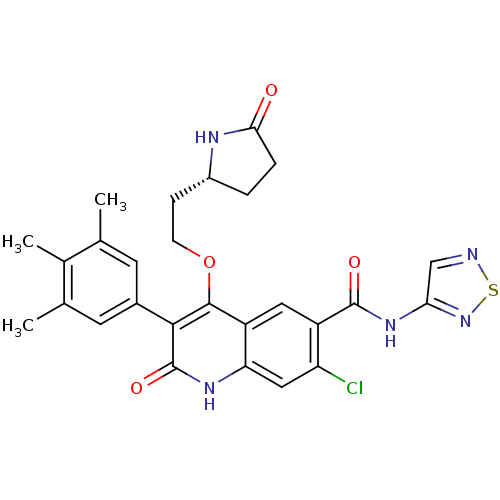
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
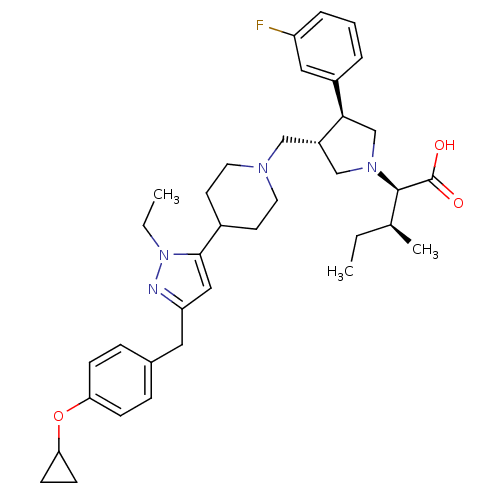
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.10E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.30E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.40E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.50E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.80E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 5.60E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 5.70E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 6.00E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 8.10E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 9.60E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.10E+4nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.40E+4nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.60E+4nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.20E+4nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.00E+4nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.70E+4nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the presence of 0.1% bovine serum albumin.More data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the presence of 0.1% bovine serum albumin.More data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Binding affinity towards human gonadotropin releasing hormone receptor expressed in CHO cells was determined by using [125I]-buserelin as radioligandMore data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the presence of 0.1% bovine serum albumin.More data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.120nMAssay Description:Inhibition GnRH-stimulated luteinizing hormone (LH) release from rat pituitary cellsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cellMore data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the presence of 0.1% bovine serum albumin.More data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the presence of 0.1% bovine serum albumin.More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cellMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cellMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cellMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cellMore data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of [125I]-buserelin binding to rat Gonadotropin-releasing hormone receptor.More data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the presence of 0.1% bovine serum albumin.More data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition GnRH-stimulated luteinizing hormone (LH) release from rat pituitary cellsMore data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the absence of bovine serum albumin (BSA)More data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition GnRH-stimulated luteinizing hormone (LH) release from rat pituitary cellsMore data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the presence of 0.1% bovine serum albumin.More data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the absence of bovine serum albumin (BSA)More data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the presence of 0.1% bovine serum albumin.More data for this Ligand-Target Pair
Affinity DataIC50: 0.240nMpH: 7.4Assay Description:In vitro inhibitory concentration against monkey plasma renin at pH 7.4More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cellMore data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Binding affinity towards human gonadotropin releasing hormone receptor expressed in CHO cells was determined by using [125I]-buserelin as radioligandMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cellMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cellMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cellMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cellMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cellMore data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the presence of 0.1% bovine serum albumin.More data for this Ligand-Target Pair