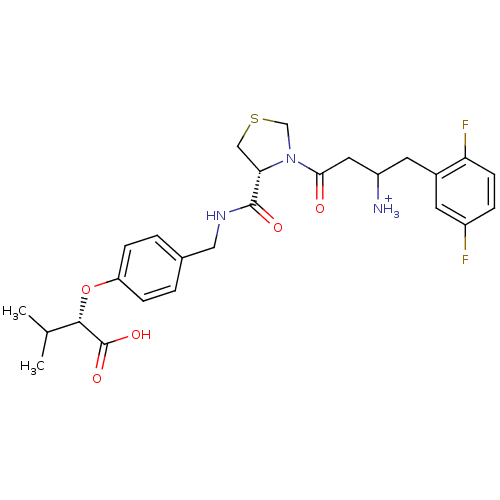
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
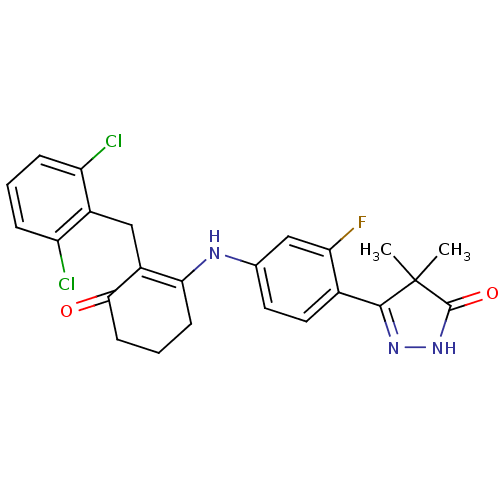
Affinity DataKi: 1.90E+3nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
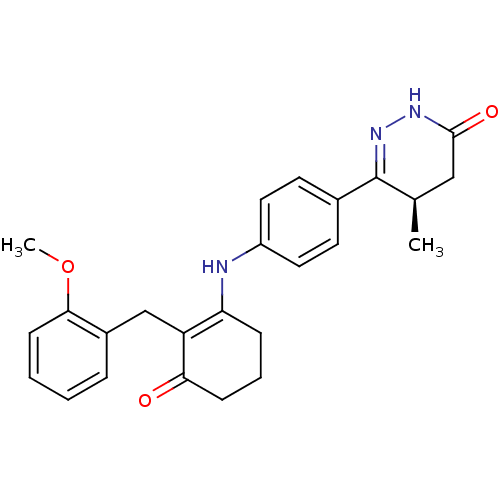
Affinity DataKi: 2.80E+3nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
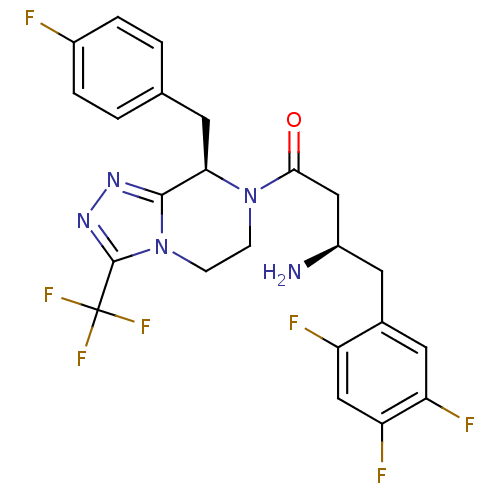
Affinity DataKi: 2.80E+3nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
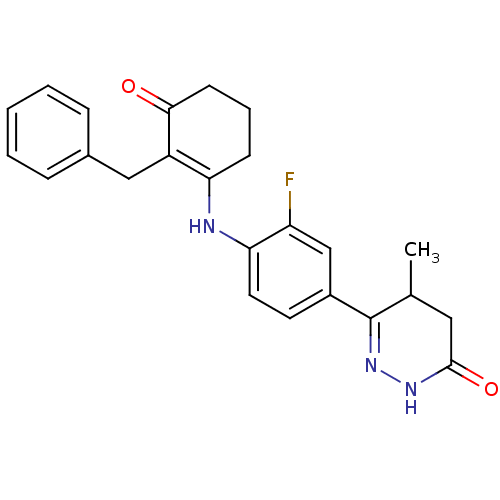
Affinity DataKi: 5.30E+3nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 5.50E+3nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 5.70E+3nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 5.70E+3nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 5.90E+3nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 5.90E+3nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 6.00E+3nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 1.60E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 1.70E+4nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 1.90E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 2.20E+4nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 2.80E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 2.90E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 3.50E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 3.50E+4nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 3.70E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 3.90E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 4.00E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 4.00E+4nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 4.20E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 4.30E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 4.90E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 5.20E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 7.30E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: >9.00E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: >9.00E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: >9.00E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [387-1112](Homo sapiens (Human))
Merck Research Laboratories
Merck Research Laboratories
Affinity DataIC50: 0.0490nMpH: 7.5 T: 2°CAssay Description:PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [387-1112](Homo sapiens (Human))
Merck Research Laboratories
Merck Research Laboratories
Affinity DataIC50: 0.0680nMpH: 7.5 T: 2°CAssay Description:PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibitory concentration against Dipeptidyl peptidase IVMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMpH: 7.5 T: 2°CAssay Description:A stable CHO (Chinese hamster ovary) cell line expressing cloned human glucagon receptor was maintained as described (Chicchi, et. al. J Biol Chem 27...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3A [388-1112](Homo sapiens (Human))
Merck Research Laboratories
Merck Research Laboratories
Affinity DataIC50: 0.110nMAssay Description:PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3A [388-1112](Homo sapiens (Human))
Merck Research Laboratories
Merck Research Laboratories
Affinity DataIC50: 0.110nMAssay Description:PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [387-1112](Homo sapiens (Human))
Merck Research Laboratories
Merck Research Laboratories
Affinity DataIC50: 0.110nMpH: 7.5 T: 2°CAssay Description:PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [387-1112](Homo sapiens (Human))
Merck Research Laboratories
Merck Research Laboratories
Affinity DataIC50: 0.130nMAssay Description:PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [387-1112](Homo sapiens (Human))
Merck Research Laboratories
Merck Research Laboratories
Affinity DataIC50: 0.130nMpH: 7.5 T: 2°CAssay Description:PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [387-1112](Homo sapiens (Human))
Merck Research Laboratories
Merck Research Laboratories
Affinity DataIC50: 0.190nMpH: 7.5 T: 2°CAssay Description:PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMpH: 7.5 T: 2°CAssay Description:A stable CHO (Chinese hamster ovary) cell line expressing cloned human glucagon receptor was maintained as described (Chicchi, et. al. J Biol Chem 27...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMpH: 7.5 T: 2°CAssay Description:A stable CHO (Chinese hamster ovary) cell line expressing cloned human glucagon receptor was maintained as described (Chicchi, et. al. J Biol Chem 27...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [387-1112](Homo sapiens (Human))
Merck Research Laboratories
Merck Research Laboratories
Affinity DataIC50: 0.240nMAssay Description:PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [387-1112](Homo sapiens (Human))
Merck Research Laboratories
Merck Research Laboratories
Affinity DataIC50: 0.260nMAssay Description:PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [654-1073](Homo sapiens (Human))
Merck Research Laboratories
Merck Research Laboratories
Affinity DataIC50: 0.270nMpH: 7.5 T: 2°CAssay Description:PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [387-1112](Homo sapiens (Human))
Merck Research Laboratories
Merck Research Laboratories
Affinity DataIC50: 0.300nMpH: 7.5 T: 2°CAssay Description:PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human recombinant Dipeptidylpeptidase IVMore data for this Ligand-Target Pair

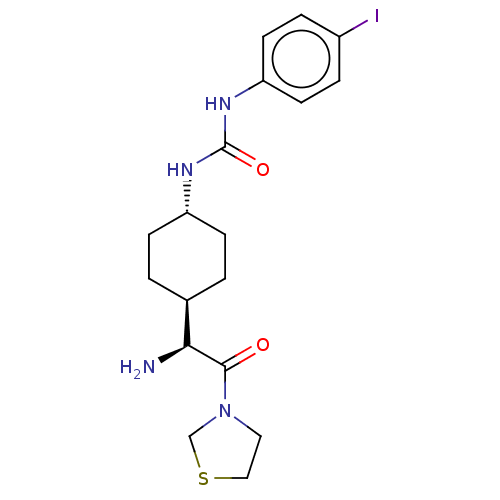

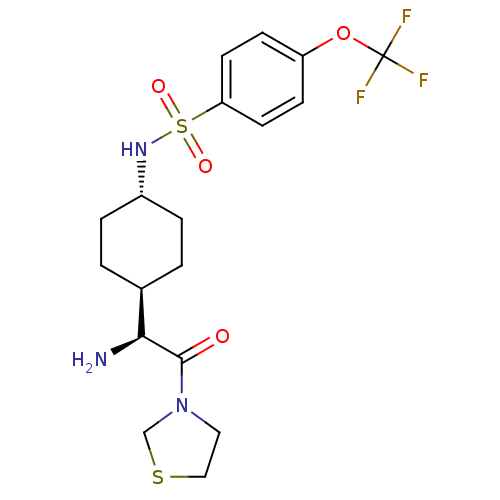
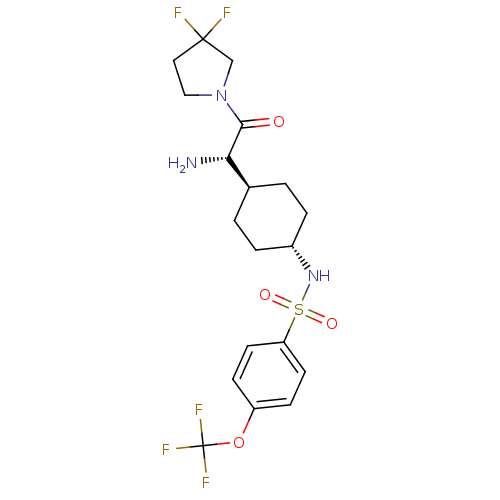
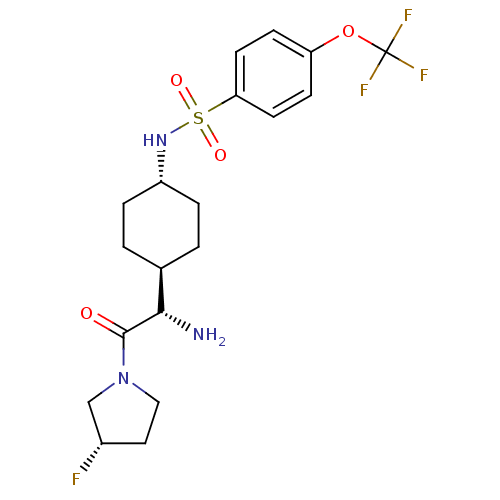
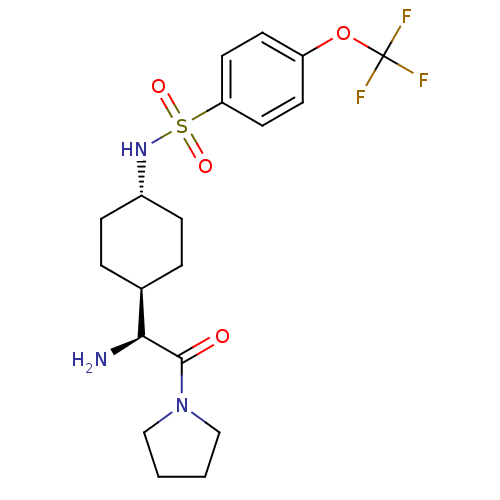

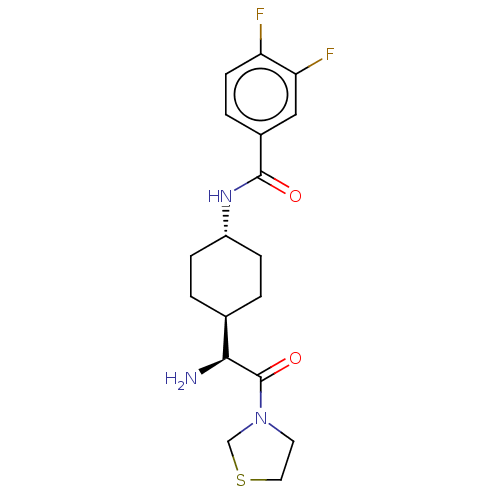
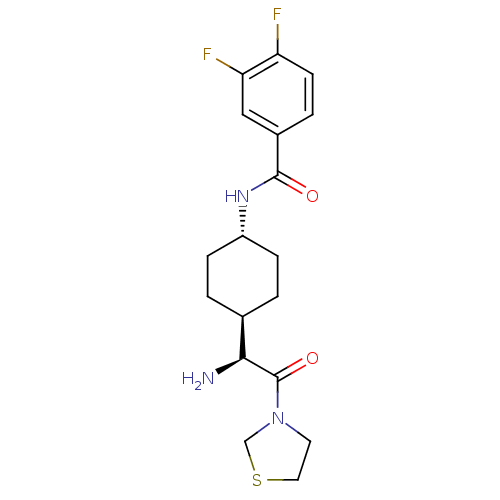
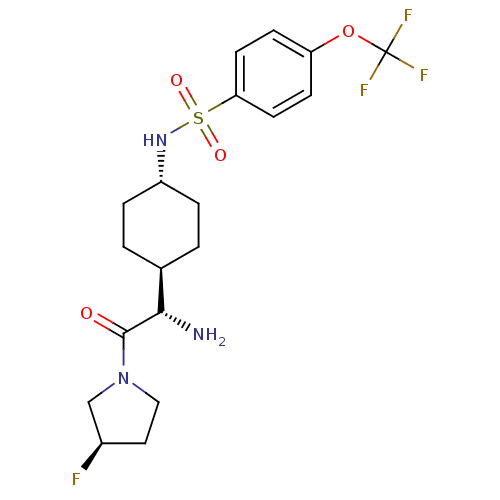
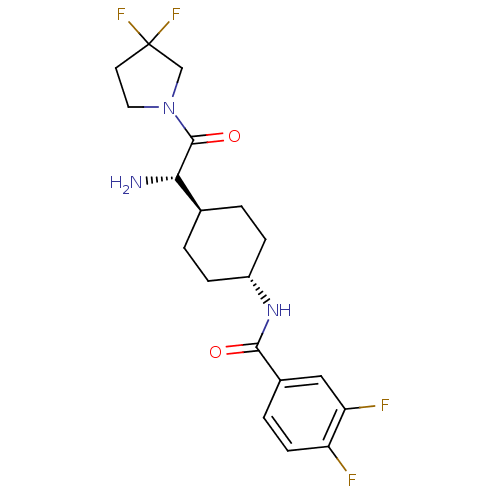

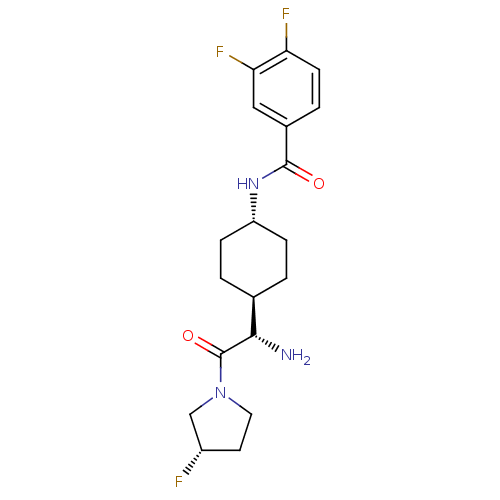

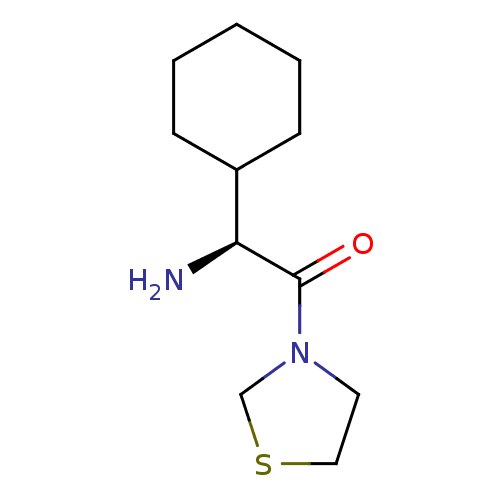
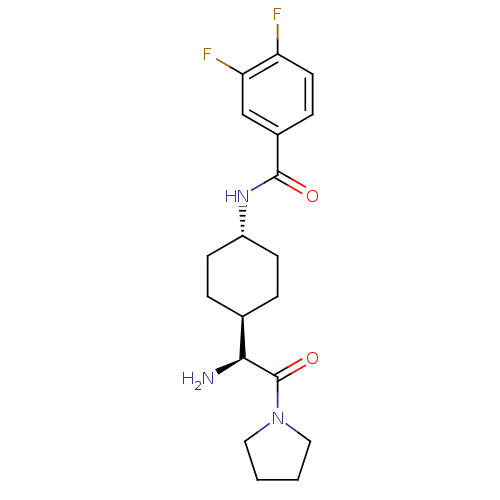
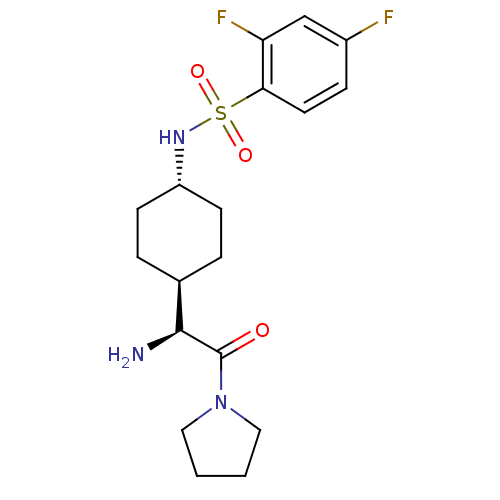
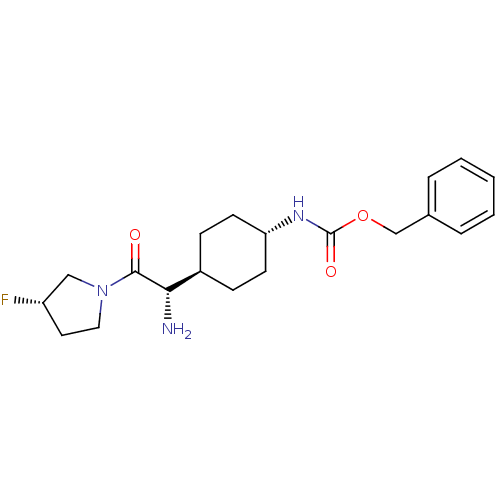
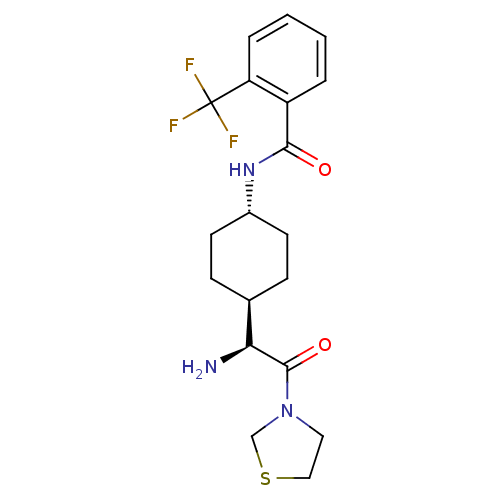
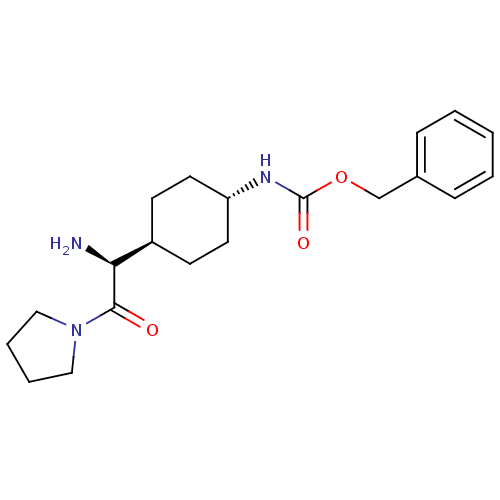
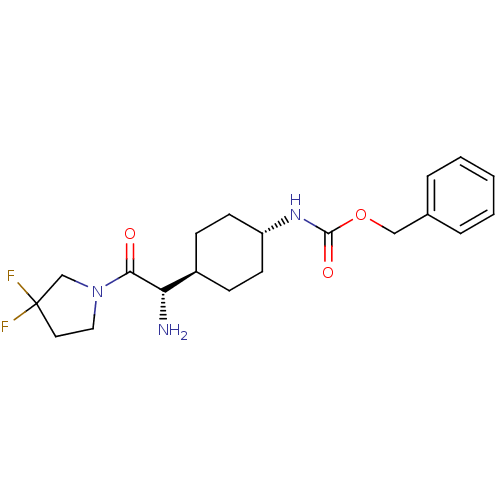
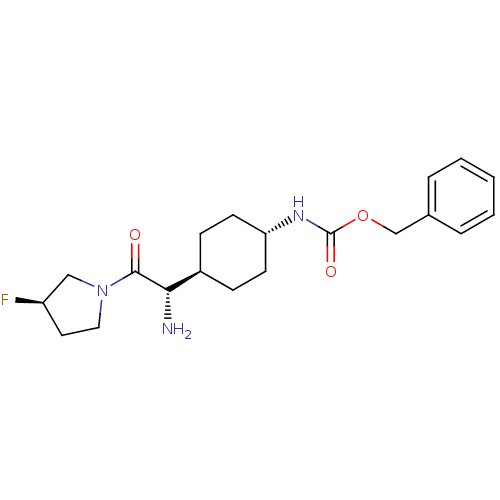
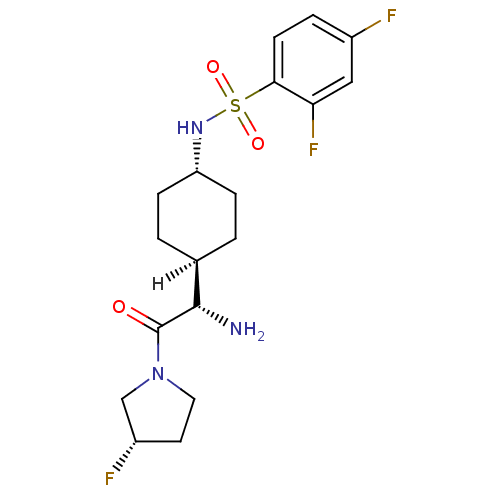
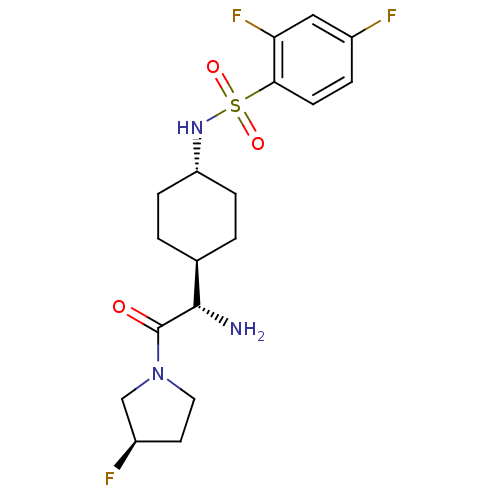
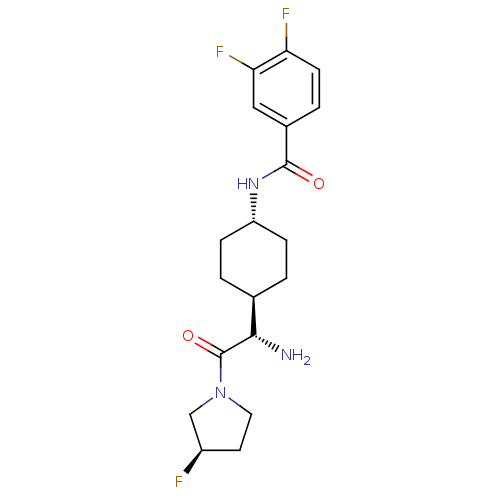
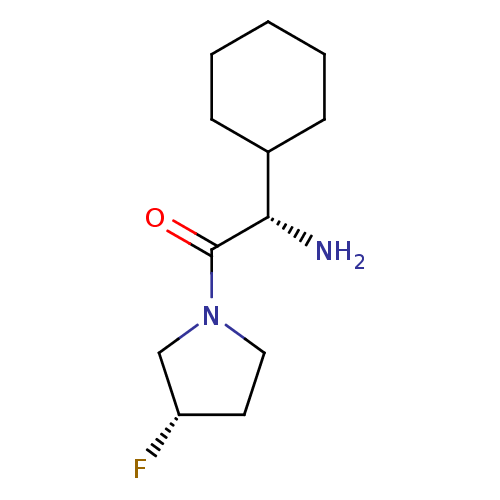
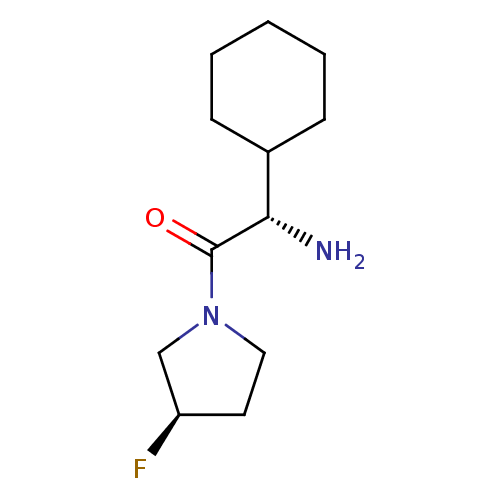
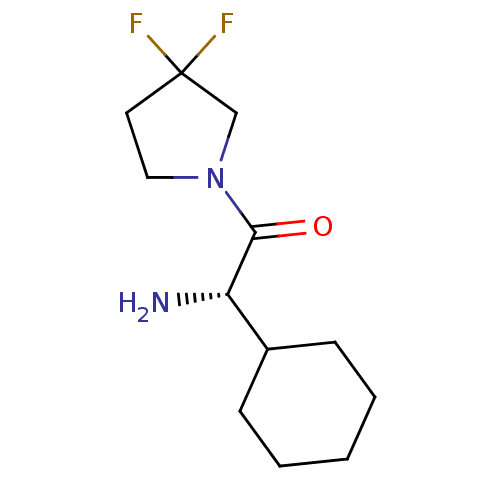
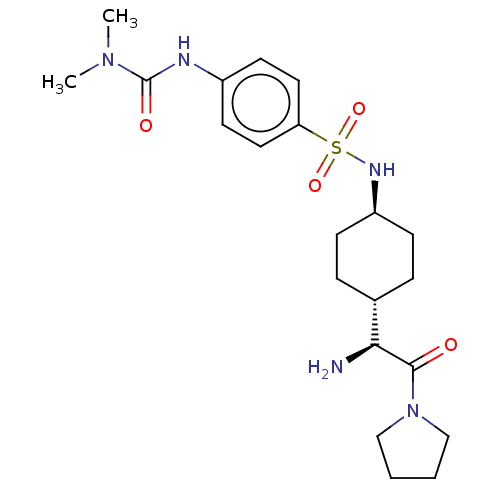
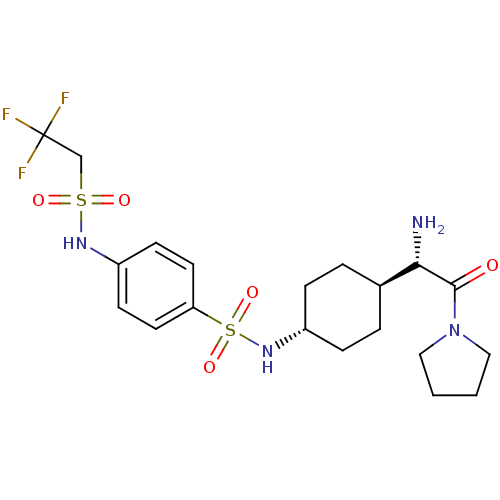

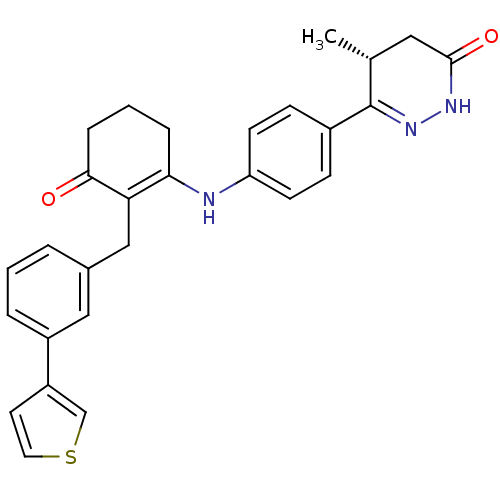
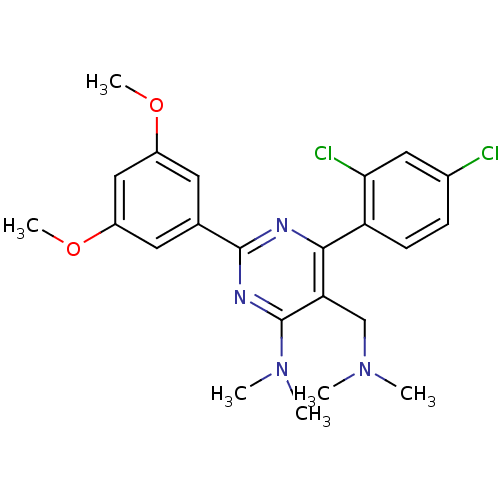

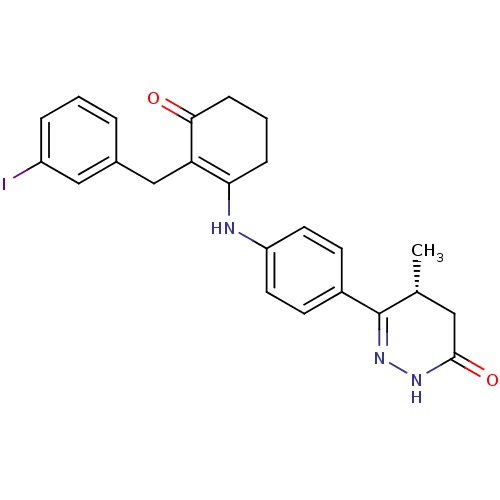
 3D Structure (crystal)
3D Structure (crystal)