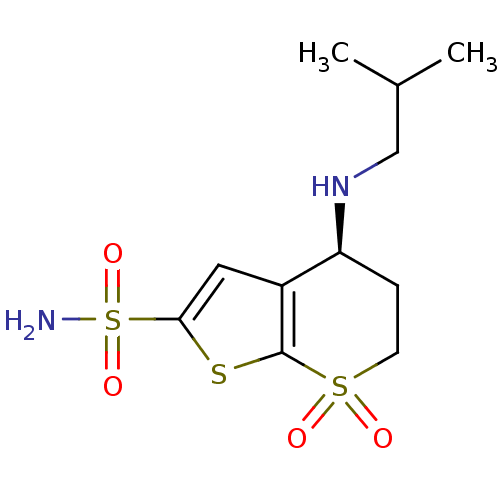
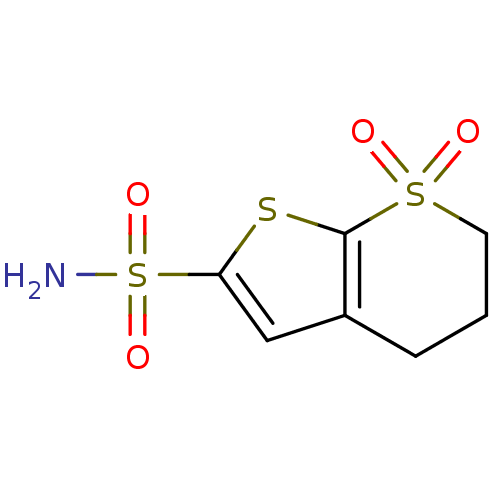
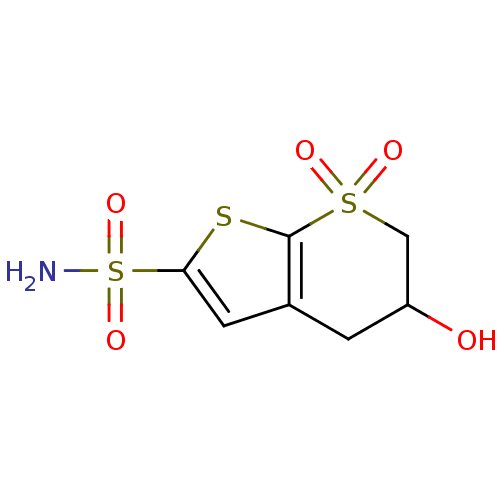
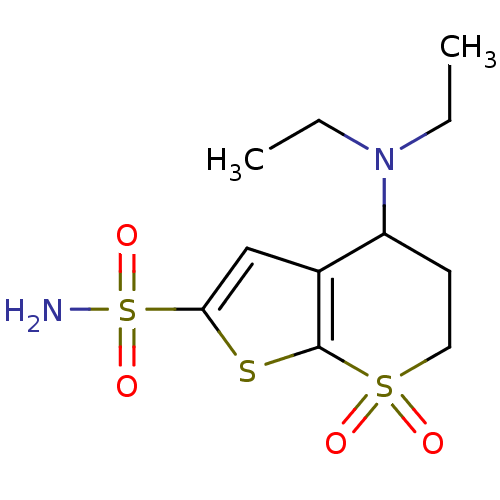
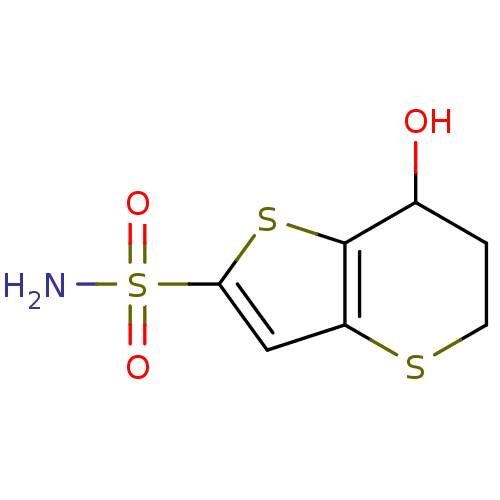
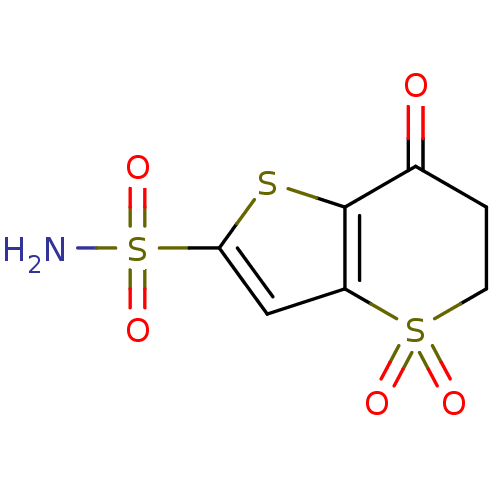
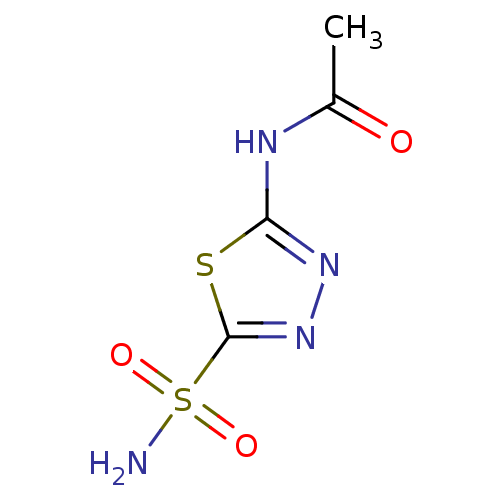
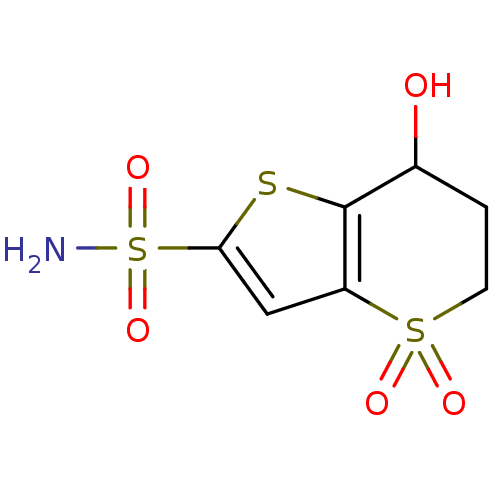
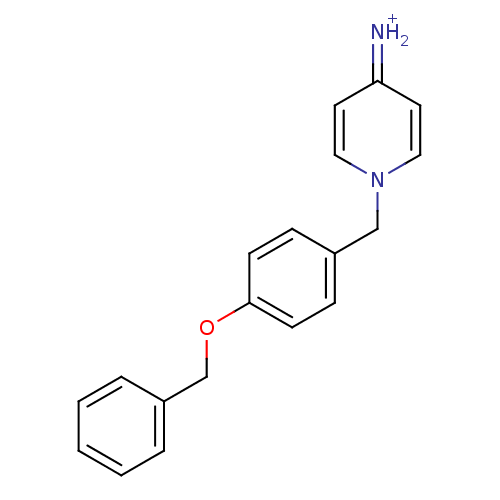
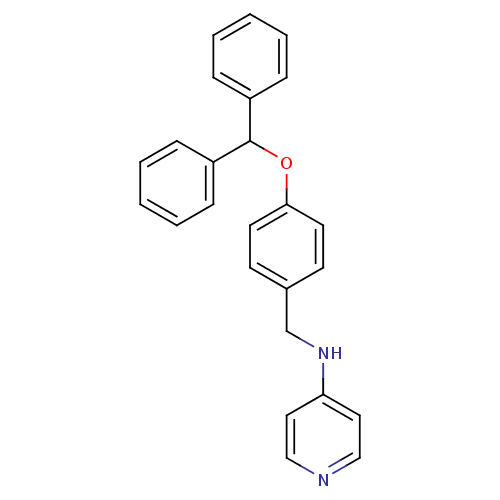
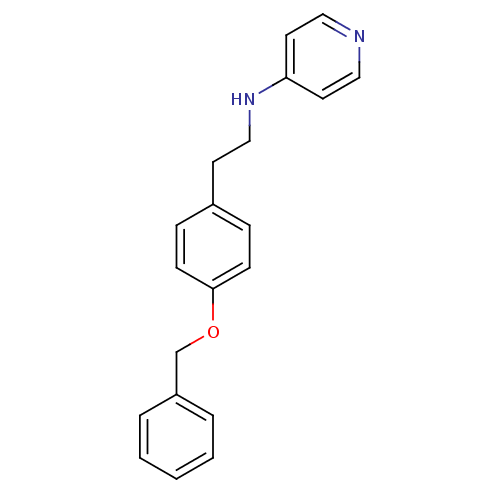
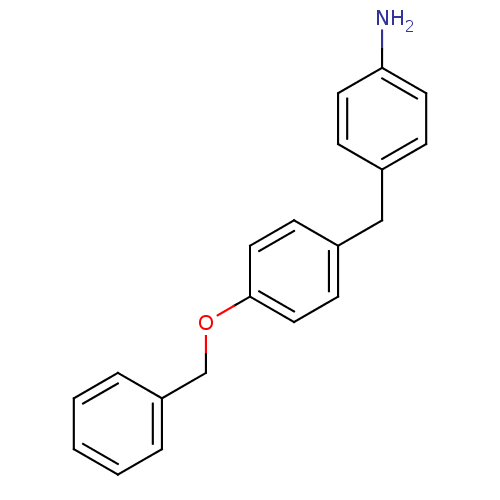
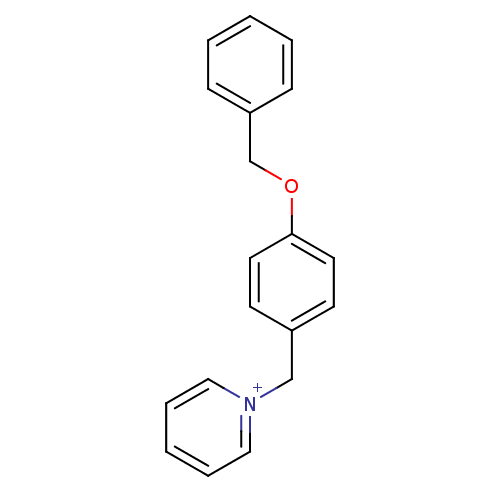
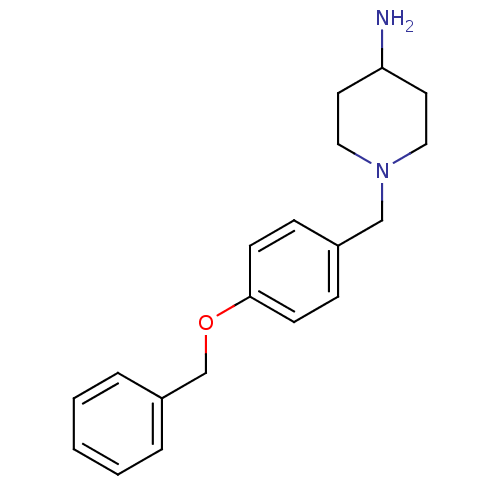
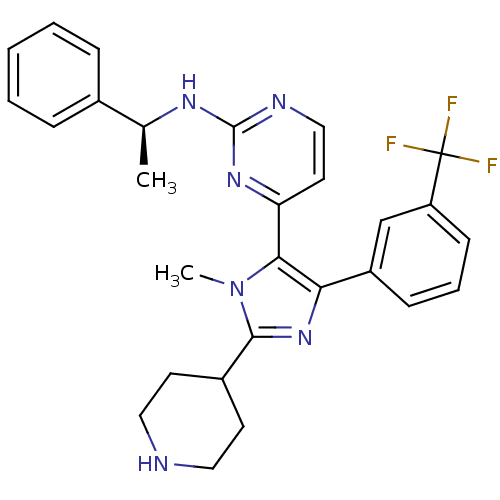
Affinity DataKi: 0.490nMAssay Description:In vitro binding to human erythrocyte carbonic anhydrase was determined by fluorescence competition assay employing the fluorescent CA inhibitor dans...More data for this Ligand-Target Pair
Affinity DataKi: 0.690nMAssay Description:The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase IIMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase IIMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:50% inhibitory concentration against human carbonic anhydrase II (HCA II) after pre-incubation for 4 min at 37 degree CMore data for this Ligand-Target Pair
Affinity DataKi: 0.820nMAssay Description:50% inhibitory concentration against human carbonic anhydrase II (HCA II) after pre-incubation at 3 degree CMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase IIMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.)More data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase IIMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase IIMore data for this Ligand-Target Pair
Affinity DataKi: 3.30nMAssay Description:In vitro binding to human erythrocyte carbonic anhydrase was determined by fluorescence competition assay employing the fluorescent CA inhibitor dans...More data for this Ligand-Target Pair
Affinity DataKi: 3.60nMAssay Description:In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.)More data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase IIMore data for this Ligand-Target Pair
Affinity DataKi: 6.20nMAssay Description:50% inhibitory concentration against human carbonic anhydrase II (HCA II) after pre-incubation for 4 min at 37 degree CMore data for this Ligand-Target Pair
Affinity DataKi: 6.70nMAssay Description:In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.)More data for this Ligand-Target Pair
Affinity DataKi: 6.80nMAssay Description:The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase IIMore data for this Ligand-Target Pair
Affinity DataKi: 7.10nMAssay Description:In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.)More data for this Ligand-Target Pair
Affinity DataKi: 8.40nMAssay Description:In vitro binding to human erythrocyte Carbonic anhydrase II was determined by fluorescence competition assay employing the fluorescent CA inhibitor d...More data for this Ligand-Target Pair
Affinity DataKi: 9.30nMAssay Description:The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase IIMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.)More data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.)More data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:In vitro binding to human erythrocyte carbonic anhydrase was determined by fluorescence competition assay employing the fluorescent CA inhibitor dans...More data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:The equilibrium dissociation constant of the inhibitor-enzyme complex of human carbonic anhydraseMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:50% inhibitory concentration against human carbonic anhydrase II (HCA II) after pre-incubation at 3 degree CMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:In vitro binding to human erythrocyte carbonic anhydrase was determined by fluorescence competition assay employing the fluorescent CA inhibitor dans...More data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:The equilibrium dissociation constant of the inhibitor-enzyme complex of human carbonic anhydraseMore data for this Ligand-Target Pair
Affinity DataKi: 28nMAssay Description:In vitro binding to human erythrocyte Carbonic anhydrase II was determined by fluorescence competition assay employing the fluorescent CA inhibitor d...More data for this Ligand-Target Pair
Affinity DataKi: 31nMAssay Description:In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.)More data for this Ligand-Target Pair
Affinity DataKi: 58nMAssay Description:Compound was evaluated for its inhibitory activity against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 71nMAssay Description:50% inhibitory concentration against human carbonic anhydrase II (HCA II) after pre-incubation for 4 min at 37 degree CMore data for this Ligand-Target Pair
Affinity DataKi: 104nMAssay Description:In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.)More data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Compound was evaluated for its inhibitory activity against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 309nMAssay Description:In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.)More data for this Ligand-Target Pair
Affinity DataKi: 700nMAssay Description:Compound was evaluated for its inhibitory activity against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.30E+3nMAssay Description:Compound was evaluated for its inhibitory activity against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 4.70E+3nMAssay Description:Compound was evaluated for its inhibitory activity against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 7.90E+3nMAssay Description:Compound was evaluated for its inhibitory activity against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Compound was evaluated for its inhibitory activity against trypsinMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Compound was evaluated for its inhibitory activity against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+4nMAssay Description:Compound was evaluated for its inhibitory activity against trypsinMore data for this Ligand-Target Pair
Affinity DataKi: 7.70E+4nMAssay Description:Compound was evaluated for its inhibitory activity against trypsinMore data for this Ligand-Target Pair
Affinity DataKi: 7.70E+4nMAssay Description:Compound was evaluated for its inhibitory activity against trypsinMore data for this Ligand-Target Pair
Affinity DataKi: 2.33E+5nMAssay Description:Compound was evaluated for its inhibitory activity against trypsinMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+5nMAssay Description:Compound was evaluated for its inhibitory activity against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 5.17E+5nMAssay Description:Compound was evaluated for its inhibitory activity against trypsinMore data for this Ligand-Target Pair
Affinity DataKi: 5.68E+5nMAssay Description:Compound was evaluated for its inhibitory activity against trypsinMore data for this Ligand-Target Pair
Affinity DataKi: 5.81E+5nMAssay Description:Compound was evaluated for its inhibitory activity against human thrombinMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.110nMAssay Description:Inhibition of Mitogen-activated protein kinase p38More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.150nMAssay Description:Inhibition of Mitogen-activated protein kinase p38More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of Mitogen-activated protein kinase p38More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of Mitogen-activated protein kinase p38More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)