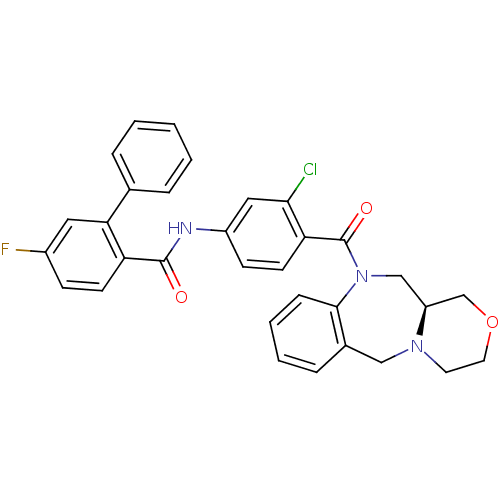
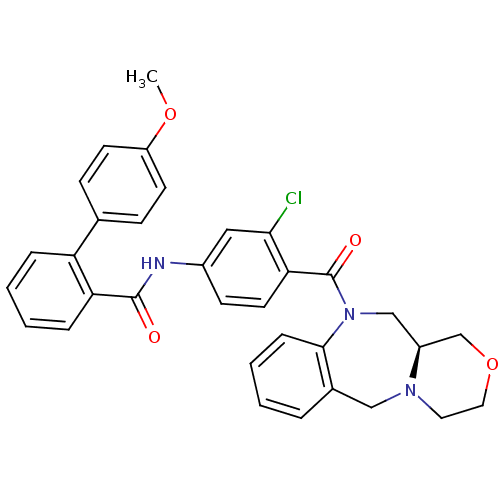
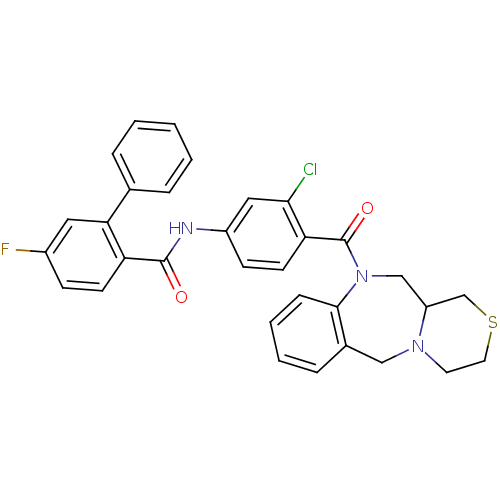
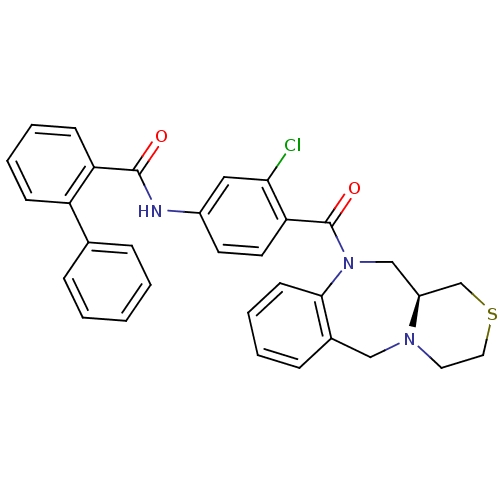
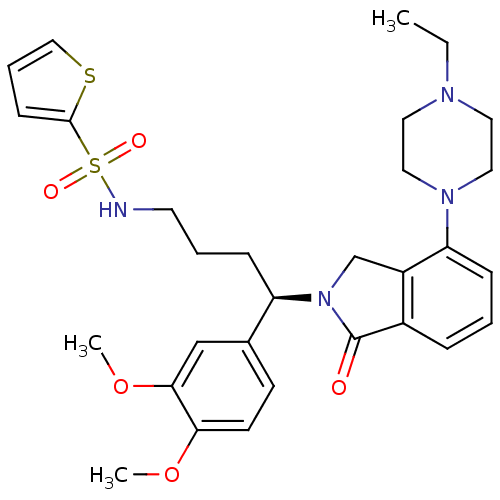
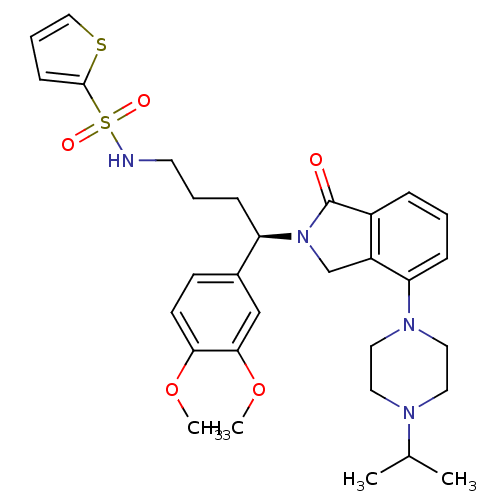
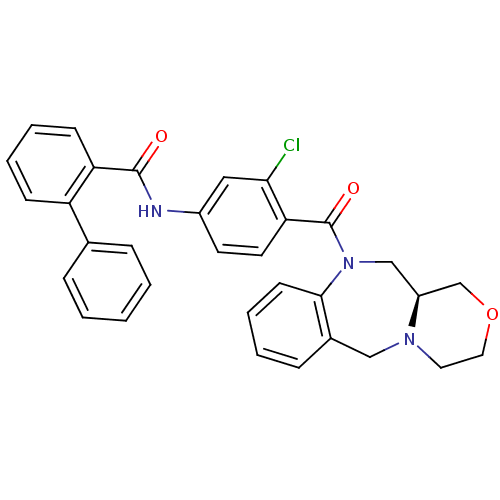
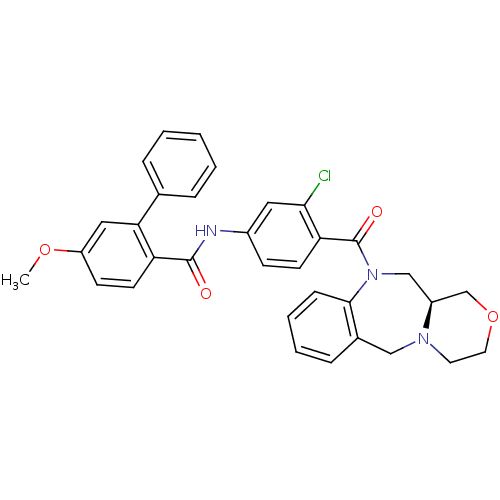
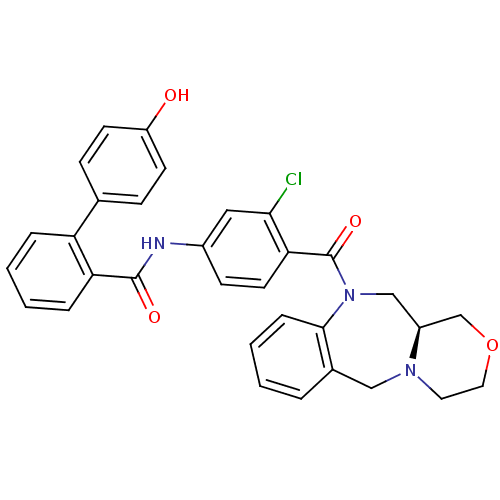
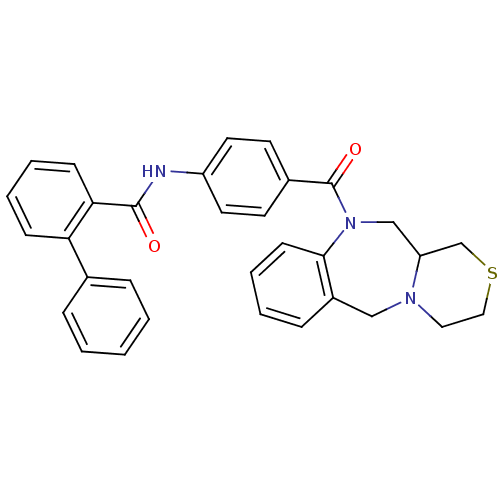
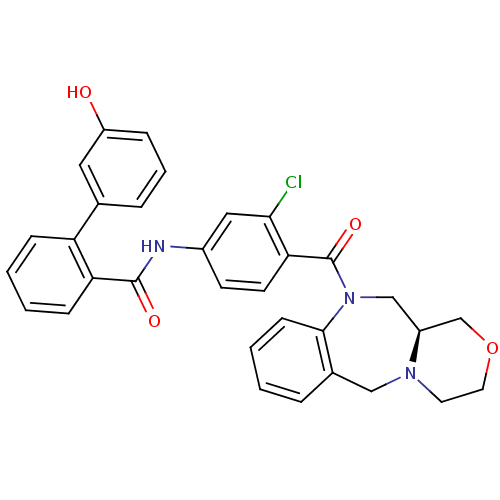
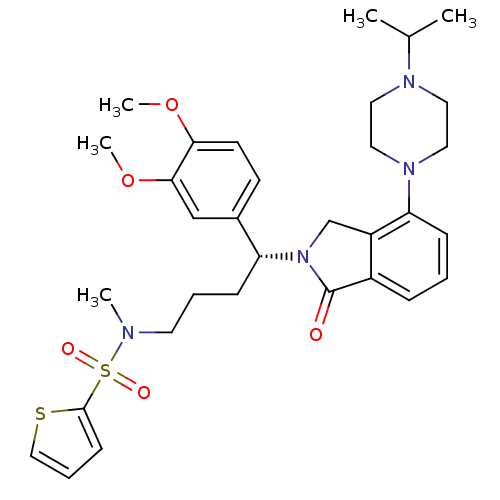
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
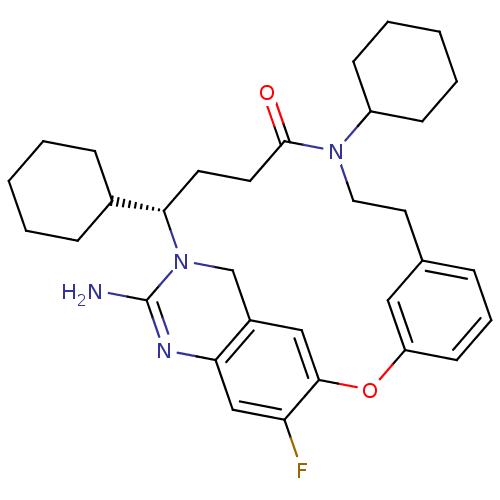
Affinity DataKi: 0.120nMAssay Description:Displacement of [125I]urotensin 2 from rat urotensin 2 receptor expressed in CHOK1 cells by scintillation proximity assayMore data for this Ligand-Target Pair
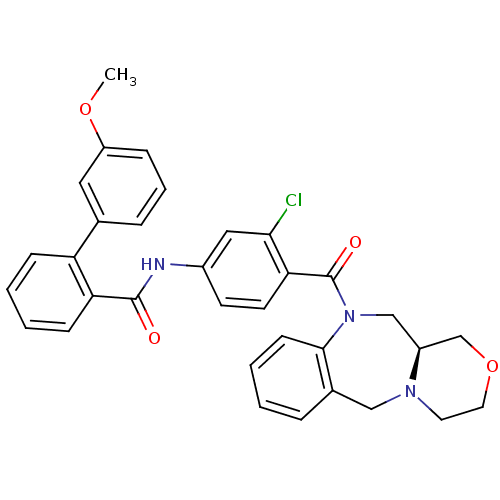
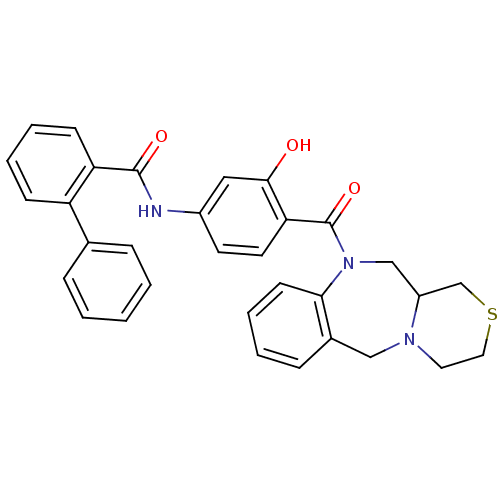
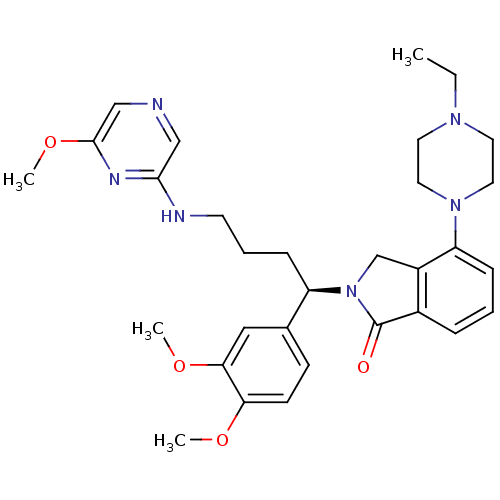
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
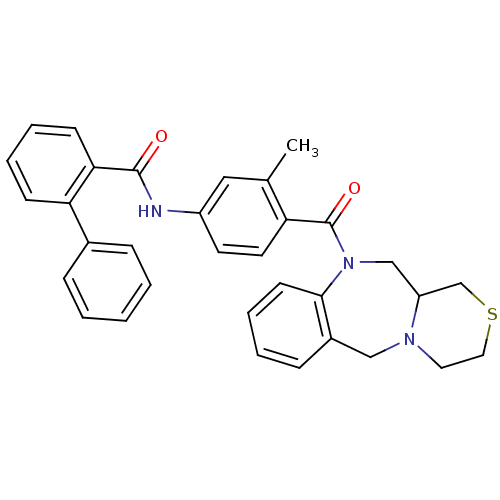
Affinity DataKi: 0.320nMAssay Description:Binding affinity against Beta-secretaseMore data for this Ligand-Target Pair
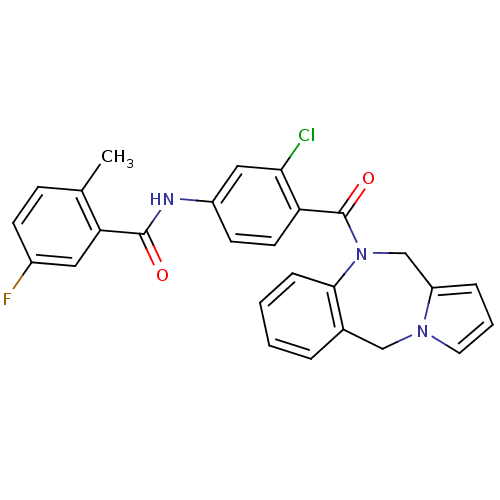
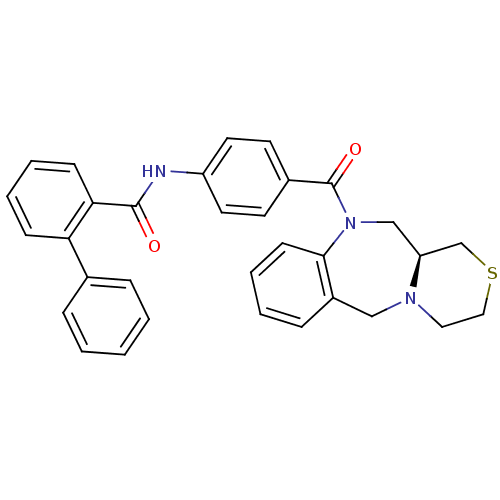
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.320nMAssay Description:Binding affinity for human brain memapsin 2 beta-Secretase (BACE)More data for this Ligand-Target Pair
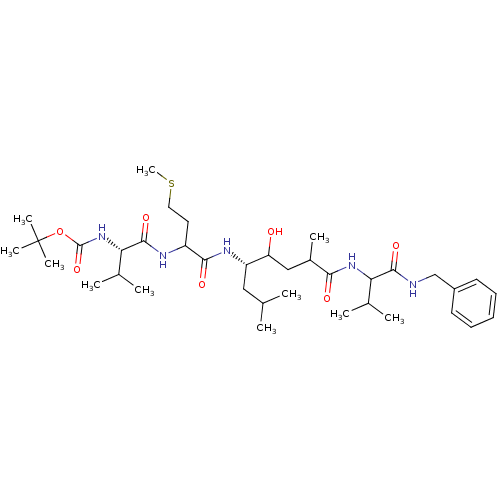
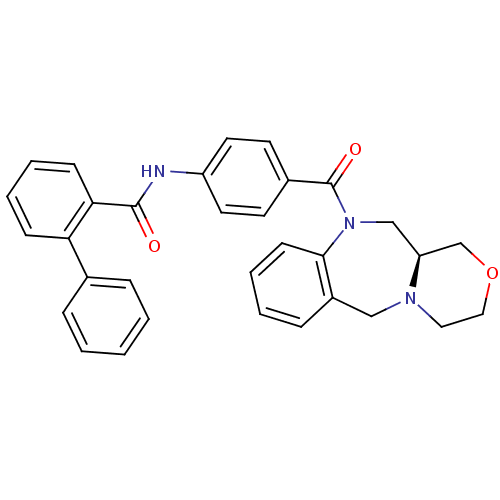
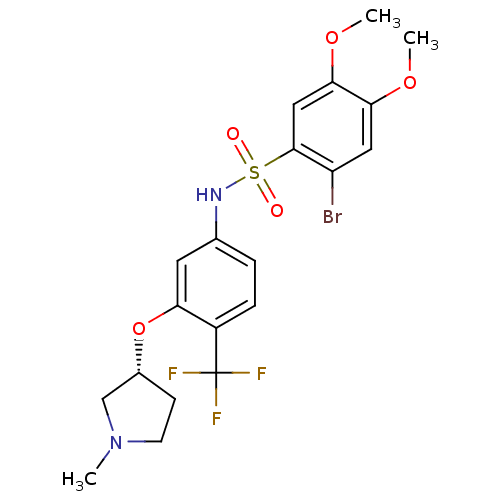
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
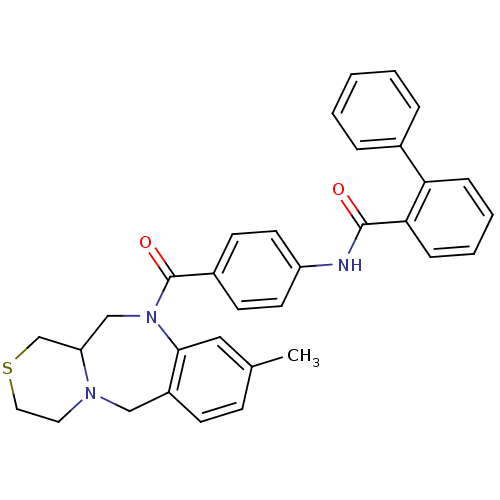
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
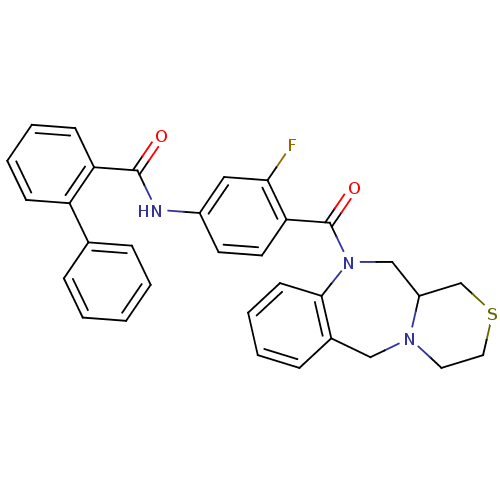
Affinity DataKi: 1.60nMAssay Description:Binding affinity for human brain memapsin 2 beta-Secretase (BACE)More data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.60nMAssay Description:Binding affinity against Beta-secretaseMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.90nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
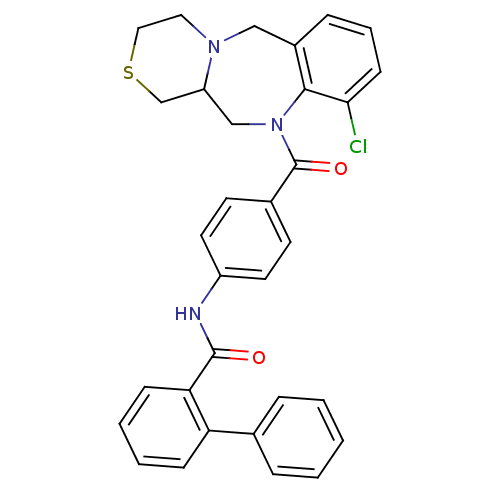
Affinity DataKi: 2.5nMAssay Description:Binding affinity for human brain memapsin 2 beta-Secretase (BACE)More data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Binding affinity against Beta-secretaseMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.80nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V1a receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assayMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assayMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4.20nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4.20nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 5.90nMAssay Description:Binding affinity for human brain memapsin 2 beta-Secretase (BACE)More data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 5.90nMAssay Description:Binding affinity against Beta-secretaseMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assayMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assayMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Binding affinity against Beta-secretaseMore data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Binding affinity for human brain memapsin 2 beta-Secretase (BACE)More data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 9.40nMAssay Description:Binding affinity against Beta-secretaseMore data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 9.40nMAssay Description:Binding affinity for human brain memapsin 2 beta-Secretase (BACE)More data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 11nM ΔG°: -45.0kJ/molepH: 5.0 T: 2°CAssay Description:BACE-1 activity was measured at pH 5 using the FS1 FRET substrate. Compounds were preincubated with recombinant BACE-1 for 20 min before adding subst...More data for this Ligand-Target Pair
TargetUrotensin-2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assayMore data for this Ligand-Target Pair

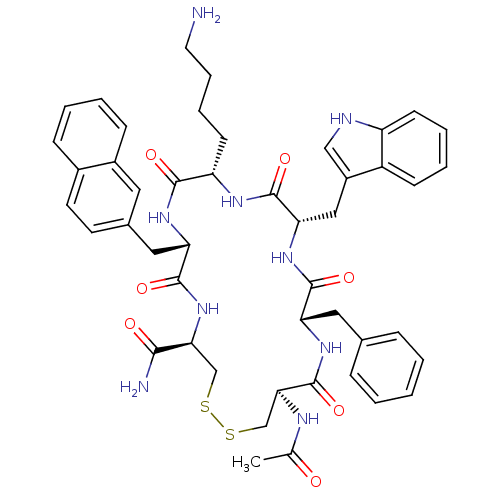
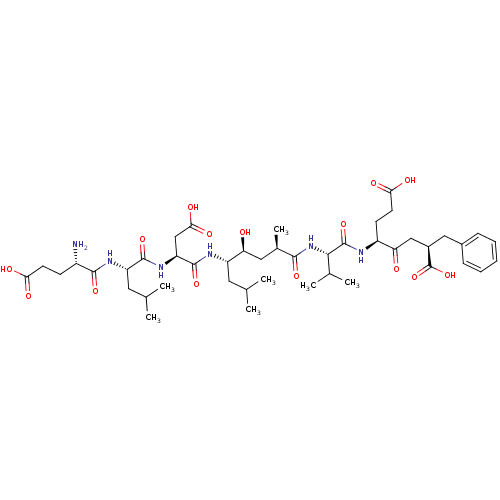
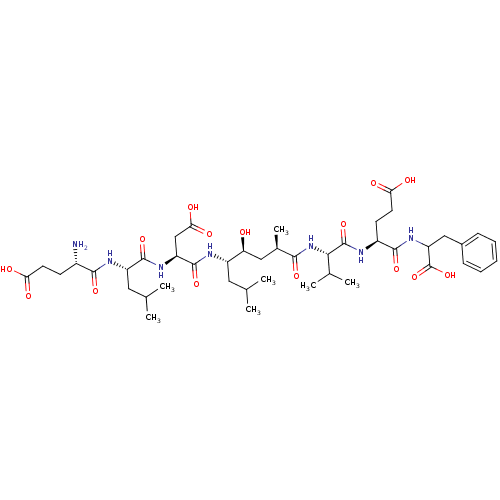
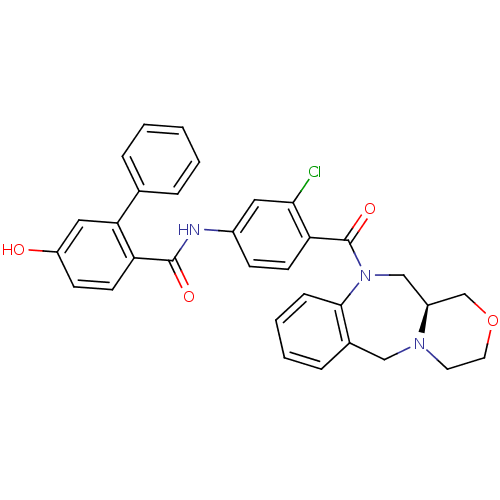
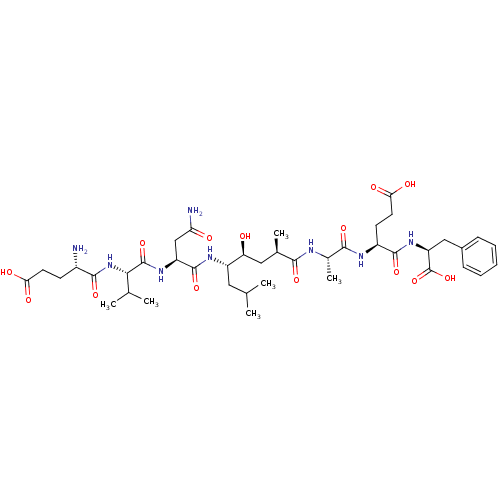
 3D Structure (crystal)
3D Structure (crystal)