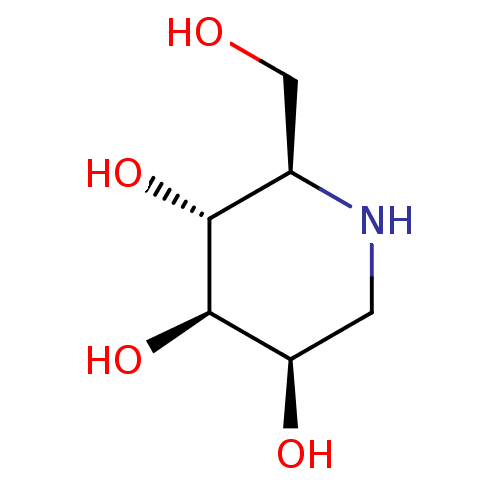
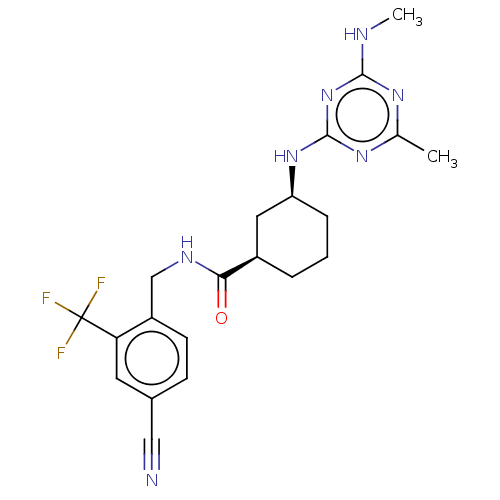
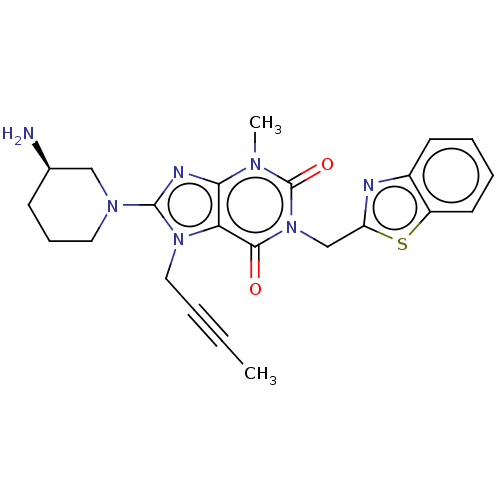
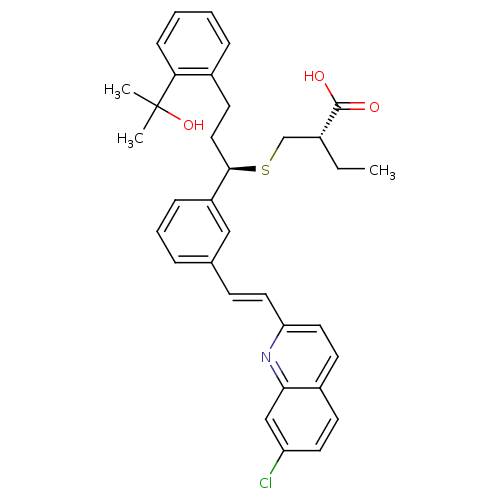
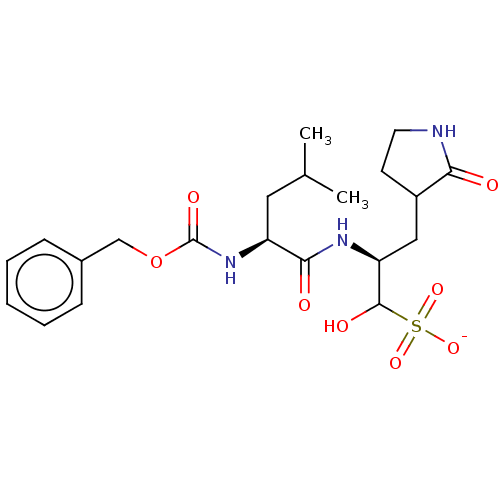
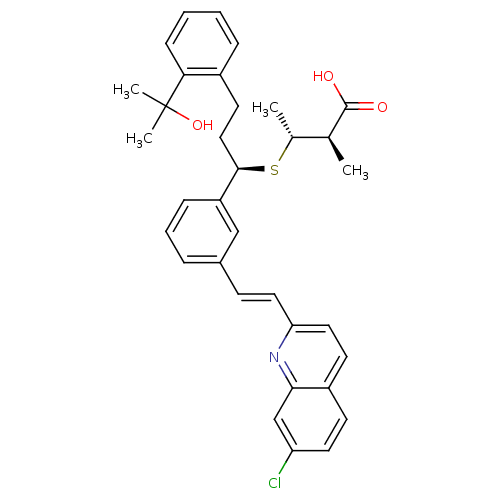
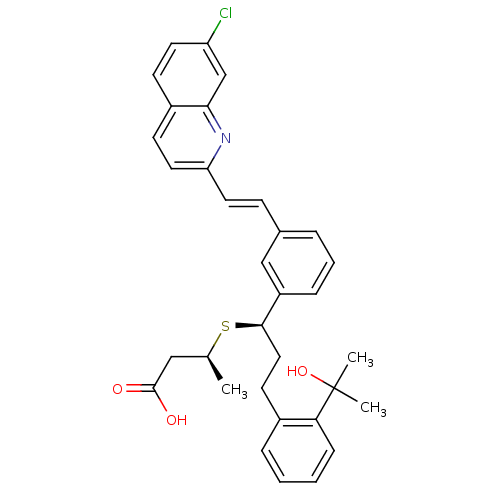
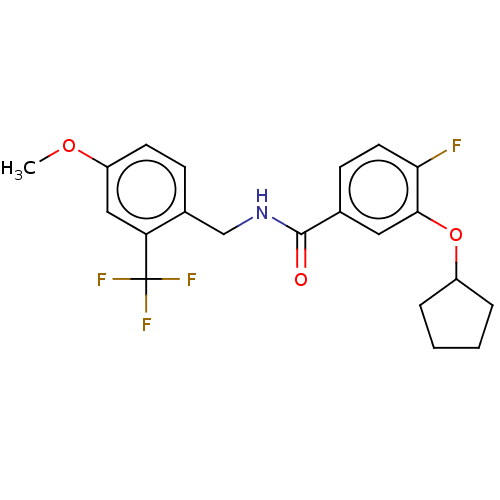
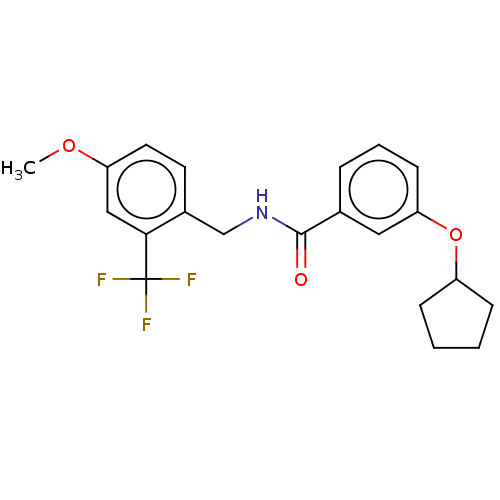
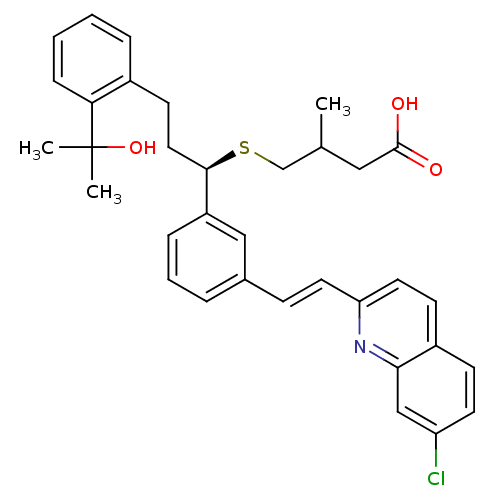
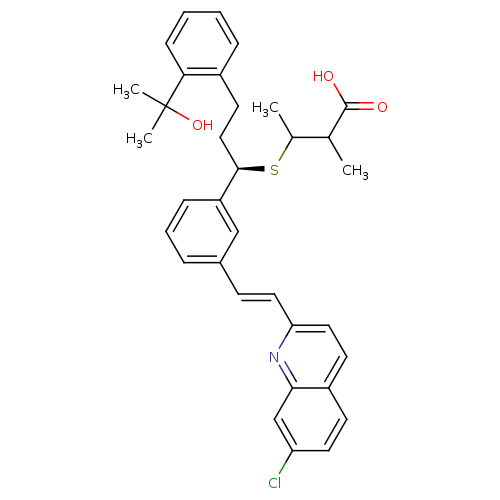
Affinity DataKi: 10nMAssay Description:Covalent binding affinity to human Bcl2A1 by FBid based FP assayMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibition of human Pim-1 using 5-FAM-RSRHSSYPAGT-CONH2 as substrate preincubated for 15 mins prior substrate addition measured after 45 mins by fluo...More data for this Ligand-Target Pair
Affinity DataKi: 31nMAssay Description:Covalent binding affinity to human Bcl2A1 by FBid based FP assayMore data for this Ligand-Target Pair
Affinity DataKi: 47nMAssay Description:Covalent binding affinity to human Bcl2A1 by FBid based FP assayMore data for this Ligand-Target Pair
Affinity DataKi: 70nMAssay Description:Non-covalent inhibition to SARS-CoV-2 -Mpro assessed as inhibition constant by native mass spectrometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 82nMAssay Description:Covalent binding affinity to human Bcl2A1 by FBid based FP assayMore data for this Ligand-Target Pair
Affinity DataKi: 133nMAssay Description:Covalent binding affinity to human Bcl2A1 by FBid based FP assayMore data for this Ligand-Target Pair
Affinity DataKi: 175nMAssay Description:Covalent binding affinity to human Bcl2A1 by FBid based FP assayMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 400nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3965 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetUrease subunit beta(Helicobacter pylori (strain ATCC 700392 / 26695) (...)
Jishou University
Curated by ChEMBL
Jishou University
Curated by ChEMBL
Affinity DataKi: 641nMAssay Description:Competitive inhibition of Helicobacter pylori ATCC 43504 urease using urea as substrate by Lineweaver-Burk/Dixon plot analysisMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3130 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Uncompetitive inhibition of human kidney glutaminase (124 to 669) assessed as reduction of glutamine hydrolysis by double-reciprocal plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.29E+3nMAssay Description:Non-covalent inhibition to SARS-CoV-2 -Mpro assessed as inhibition constant by native mass spectrometric analysisMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 5.00E+3nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3990 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 1.40E+4nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2199 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 4.30E+4nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3994 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 4.60E+4nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3991 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 7.00E+4nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2948 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 9.00E+4nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2199 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 9.60E+4nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3990 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 1.40E+5nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3990 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 2.30E+5nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2199 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 2.60E+5nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3858 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 2.70E+5nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3962 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 3.60E+5nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 4073 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase/Alpha-1,2-mannosidase family protein/Alpha-1,2-mannosidase, putative/Glycoside hydrolase family 92/Putative alpha-1,2-mannosidase(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 6.00E+5nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 1032 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 7.90E+5nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3963 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 8.60E+5nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 4093 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 1.60E+6nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 4092 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 1.80E+6nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 1878 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 5.80E+6nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3784 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 1.20E+7nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3990 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetAlpha-1,2-mannosidase, putative(Bacteroides thetaiotaomicron )
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataKi: 1.30E+7nMAssay Description:Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 2199 assessed as reduction of mannose release using 4NP-mannopyranoside substrateMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.0270nMAssay Description:Inhibition of recombinant human sEH using MNPC as substrate by fluorescence-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0500nMT: 2°CAssay Description:Gly-Pro-7-amido-4-methylcoumarin can be hydrolyzed by dipeptidyl peptidase IV (DPP-IV) at room temperature, to generate 7-amido-4-methyl coumarin, wh...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0800nMT: 2°CAssay Description:Gly-Pro-7-amido-4-methylcoumarin can be hydrolyzed by dipeptidyl peptidase IV (DPP-IV) at room temperature, to generate 7-amido-4-methyl coumarin, wh...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMT: 2°CAssay Description:Gly-Pro-7-amido-4-methylcoumarin can be hydrolyzed by dipeptidyl peptidase IV (DPP-IV) at room temperature, to generate 7-amido-4-methyl coumarin, wh...More data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMT: 2°CAssay Description:Gly-Pro-7-amido-4-methylcoumarin can be hydrolyzed by dipeptidyl peptidase IV (DPP-IV) at room temperature, to generate 7-amido-4-methyl coumarin, wh...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Concentration required for inhibition of binding of [3H]-LTD4 to guinea pig lung membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMT: 2°CAssay Description:Gly-Pro-7-amido-4-methylcoumarin can be hydrolyzed by dipeptidyl peptidase IV (DPP-IV) at room temperature, to generate 7-amido-4-methyl coumarin, wh...More data for this Ligand-Target Pair
Affinity DataIC50: 0.210nMAssay Description:FRET-based enzymatic assay.More data for this Ligand-Target Pair
Affinity DataIC50: 0.260nMAssay Description:Enzymatic inhibition of GC-376, UAWJ9-36-1, and UAWJ9-36-3 against Mpro's from all seven human coronaviruses. Data fittings of the proteolytic progre...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Concentration required for inhibition of binding of [3H]-LTD4 to guinea pig lung membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Concentration required for inhibition of binding of [3H]-LTD4 to guinea pig lung membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.330nMAssay Description:Concentration required for inhibition of binding of [3H]-LTD4 to guinea pig lung membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.380nMAssay Description:Inhibition of binding of [ H]-LTD4 to DMSO differentiated U937 cell membranesMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human sEH using MNPC as substrate by fluorescence-based assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human sEH using MNPC as substrate by fluorescence-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMAssay Description:Concentration required for inhibition of binding of [3H]-LTD4 to guinea pig lung membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.440nMAssay Description:Concentration required for inhibition of binding of [3H]-LTD4 to guinea pig lung membranesMore data for this Ligand-Target Pair





 3D Structure (crystal)
3D Structure (crystal)