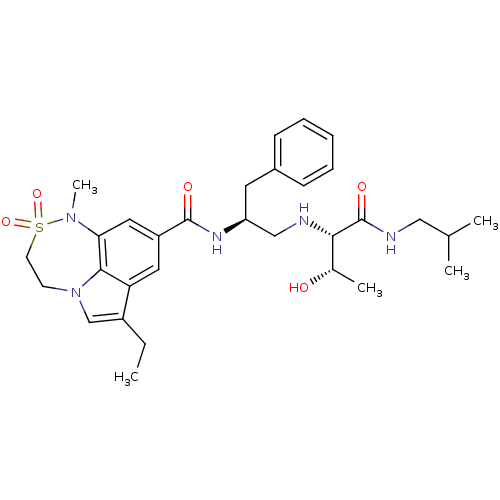
Affinity DataKi: 0.0170nMAssay Description:Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f...More data for this Ligand-Target Pair
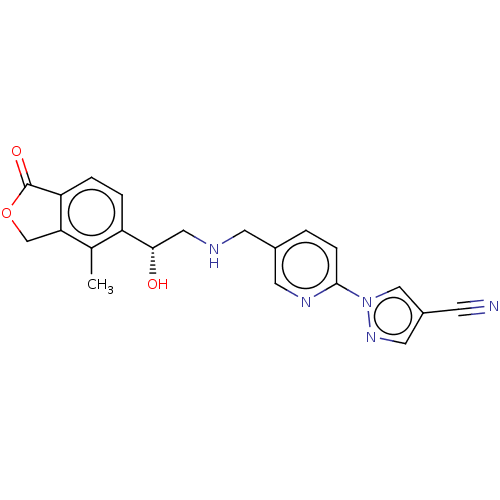
Affinity DataKi: 0.0360nMAssay Description:Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f...More data for this Ligand-Target Pair
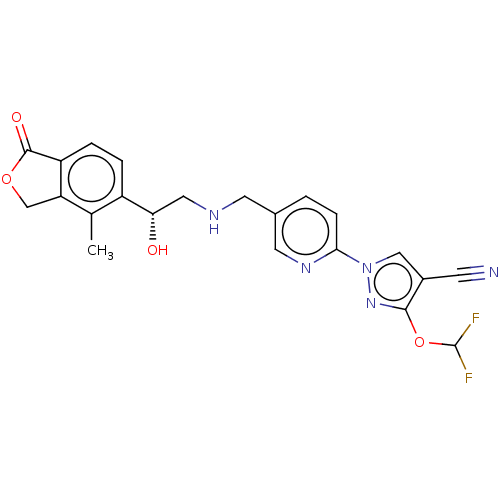
Affinity DataKi: 0.400nMAssay Description:Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f...More data for this Ligand-Target Pair
Affinity DataKi: 0.470nMAssay Description:Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f...More data for this Ligand-Target Pair
Affinity DataKi: 7.20nMAssay Description:Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f...More data for this Ligand-Target Pair
Affinity DataKi: 7.30nMAssay Description:Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f...More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibition of recombinant BACE2More data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f...More data for this Ligand-Target Pair
Affinity DataKi: 27nMAssay Description:Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f...More data for this Ligand-Target Pair
Affinity DataKi: 99nMAssay Description:Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f...More data for this Ligand-Target Pair
Affinity DataKi: 120nMAssay Description:Inhibition of recombinant BACE2More data for this Ligand-Target Pair
Affinity DataKi: 530nMAssay Description:Inhibition of human cathepsin DMore data for this Ligand-Target Pair
Affinity DataKi: 690nMAssay Description:Inhibition of human cathepsin DMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f...More data for this Ligand-Target Pair
Affinity DataKi: 1.45E+3nMAssay Description:Inhibition of recombinant BACE2More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of human cathepsin DMore data for this Ligand-Target Pair
Affinity DataKi: 4.30E+3nMAssay Description:Inhibition of human cathepsin DMore data for this Ligand-Target Pair
Affinity DataKi: 7.10E+3nMAssay Description:Inhibition of human cathepsin DMore data for this Ligand-Target Pair
Affinity DataKi: 8.26E+3nMAssay Description:Inhibition of human cathepsin DMore data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 1(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 0.150nMAssay Description:The coverslip plated with cells was placed in the experiment chamber perfused with bath solution composed of (in mM): 135 NaCl, 5 KCl, 2 CaCl2), 1 Mg...More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 1(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 0.570nMAssay Description:Solutions and reagents: Thallium flux assay was performed using FluxOR kit (F10017, Life Technologies). Loading buffer, assay buffer and stimulus buf...More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 1(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 1nMAssay Description:Solutions and reagents: Thallium flux assay was performed using FluxOR kit (F10017, Life Technologies). Loading buffer, assay buffer and stimulus buf...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of BACE1 in human neuroblastoma cellsMore data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 1(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 1.10nMAssay Description:The coverslip plated with cells was placed in the experiment chamber perfused with bath solution composed of (in mM): 135 NaCl, 5 KCl, 2 CaCl2), 1 Mg...More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 1(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 2.10nMAssay Description:The coverslip plated with cells was placed in the experiment chamber perfused with bath solution composed of (in mM): 135 NaCl, 5 KCl, 2 CaCl2), 1 Mg...More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 1(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 2.40nMAssay Description:Solutions and reagents: Thallium flux assay was performed using FluxOR kit (F10017, Life Technologies). Loading buffer, assay buffer and stimulus buf...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Inhibition of BACE1 in human neuroblastoma cellsMore data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 1(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 3.60nMAssay Description:Solutions and reagents: Thallium flux assay was performed using FluxOR kit (F10017, Life Technologies). Loading buffer, assay buffer and stimulus buf...More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 1(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 3.60nMAssay Description:Solutions and reagents: Thallium flux assay was performed using FluxOR kit (F10017, Life Technologies). Loading buffer, assay buffer and stimulus buf...More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 1(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 4nMAssay Description:Solutions and reagents: Thallium flux assay was performed using FluxOR kit (F10017, Life Technologies). Loading buffer, assay buffer and stimulus buf...More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 1(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 4.70nMAssay Description:The coverslip plated with cells was placed in the experiment chamber perfused with bath solution composed of (in mM): 135 NaCl, 5 KCl, 2 CaCl2), 1 Mg...More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 1(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 4.70nMAssay Description:The coverslip plated with cells was placed in the experiment chamber perfused with bath solution composed of (in mM): 135 NaCl, 5 KCl, 2 CaCl2, 1 MgC...More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 1(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 4.80nMAssay Description:The coverslip plated with cells was placed in the experiment chamber perfused with bath solution composed of (in mM): 135 NaCl, 5 KCl, 2 CaCl2, 1 MgC...More data for this Ligand-Target Pair
TargetATP-sensitive inward rectifier potassium channel 1(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 4.90nMAssay Description:The coverslip plated with cells was placed in the experiment chamber perfused with bath solution composed of (in mM): 135 NaCl, 5 KCl, 2 CaCl2, 1 MgC...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.5Assay Description:The effectiveness of compounds of the present invention as ROCK inhibitors can be determined in a 30 μL assay containing 20 mM HEPES, pH 7.5, 20...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.5Assay Description:The effectiveness of compounds of the present invention as ROCK inhibitors can be determined in a 30 μL assay containing 20 mM HEPES, pH 7.5, 20...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.5Assay Description:The effectiveness of compounds of the present invention as ROCK inhibitors can be determined in a 30 μL assay containing 20 mM HEPES, pH 7.5, 20...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.5Assay Description:The effectiveness of compounds of the present invention as ROCK inhibitors can be determined in a 30 μL assay containing 20 mM HEPES, pH 7.5, 20...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.5Assay Description:The effectiveness of compounds of the present invention as ROCK inhibitors can be determined in a 30 μL assay containing 20 mM HEPES, pH 7.5, 20...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.5Assay Description:The effectiveness of compounds of the present invention as ROCK inhibitors can be determined in a 30 μL assay containing 20 mM HEPES, pH 7.5, 20...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.5Assay Description:The effectiveness of compounds of the present invention as ROCK inhibitors can be determined in a 30 μL assay containing 20 mM HEPES, pH 7.5, 20...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.5Assay Description:The effectiveness of compounds of the present invention as ROCK inhibitors can be determined in a 30 μL assay containing 20 mM HEPES, pH 7.5, 20...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.5Assay Description:The effectiveness of compounds of the present invention as ROCK inhibitors can be determined in a 30 μL assay containing 20 mM HEPES, pH 7.5, 20...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.5Assay Description:The effectiveness of compounds of the present invention as ROCK inhibitors can be determined in a 30 μL assay containing 20 mM HEPES, pH 7.5, 20...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.5Assay Description:The effectiveness of compounds of the present invention as ROCK inhibitors can be determined in a 30 μL assay containing 20 mM HEPES, pH 7.5, 20...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.5Assay Description:The effectiveness of compounds of the present invention as ROCK inhibitors can be determined in a 30 μL assay containing 20 mM HEPES, pH 7.5, 20...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.5Assay Description:The effectiveness of compounds of the present invention as ROCK inhibitors can be determined in a 30 μL assay containing 20 mM HEPES, pH 7.5, 20...More data for this Ligand-Target Pair

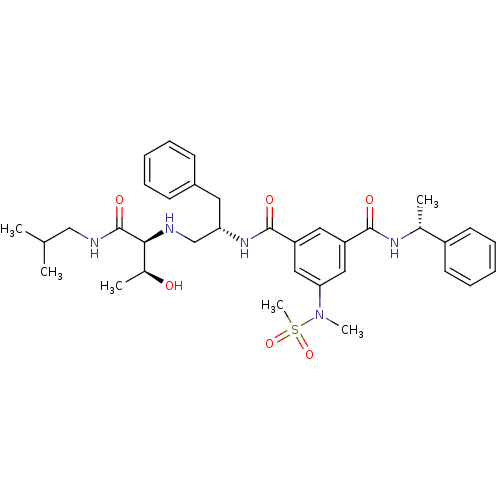
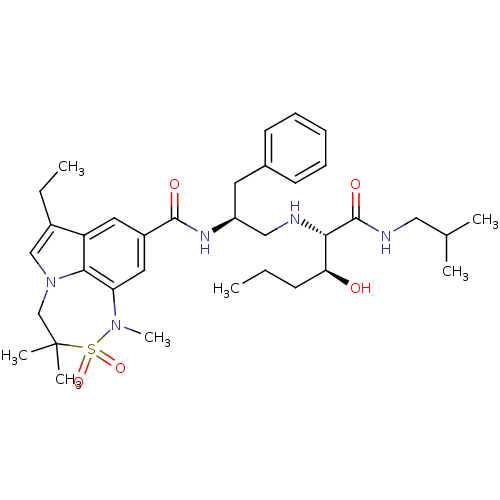
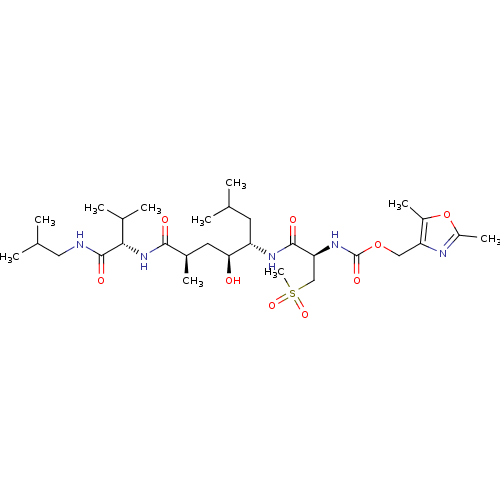
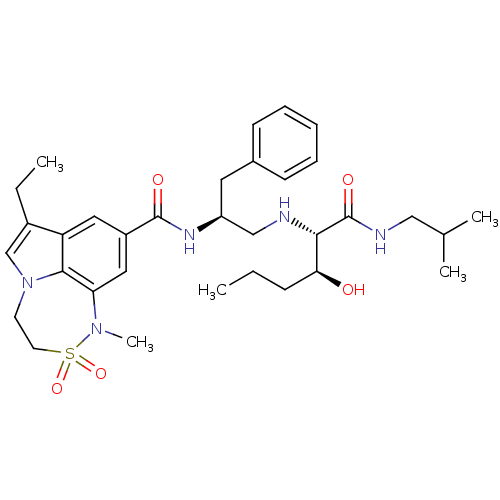
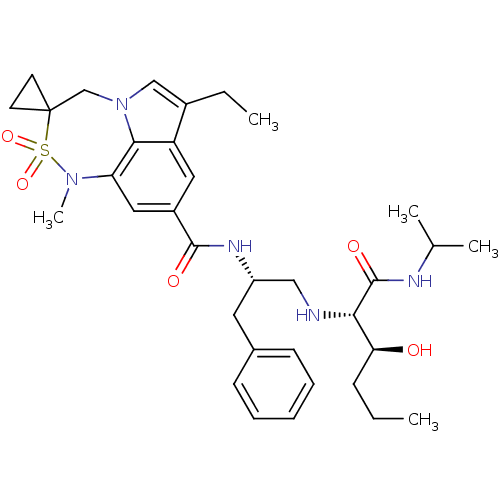
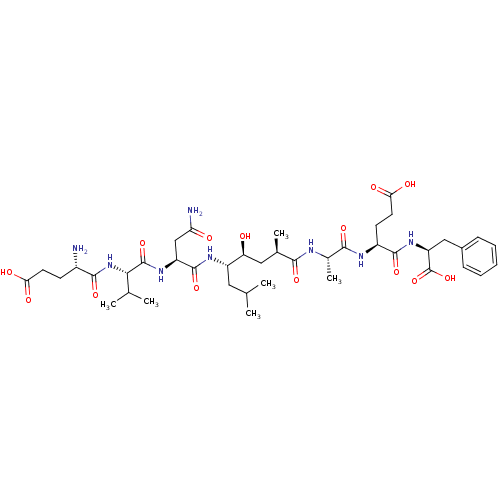
 3D Structure (crystal)
3D Structure (crystal)