Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB (change energy unit to kcal/mol)
 Found 286 hits in this display
Found 286 hits in this display
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
 BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)
BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)
 BDBM82124(adenosine-derived inhibitor (Grp78), 1)
BDBM82124(adenosine-derived inhibitor (Grp78), 1)
 BDBM50248004(CHEBI:63599 | Fludara | Fludarabine | Fludarabine ...)
BDBM50248004(CHEBI:63599 | Fludara | Fludarabine | Fludarabine ...)Ki: 131nMAssay Description:Inhibition of recombinant human carbonic anhydrase VA preincubated with enzyme for 15 mins by phenol red dye based stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
 BDBM81592(AppppA analog, 17 (X=O))
BDBM81592(AppppA analog, 17 (X=O))
 BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)
BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)
 BDBM81587(AppppA analog, 14 (X=O))
BDBM81587(AppppA analog, 14 (X=O))
 BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)
BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM81583(AppppA analog, 12 (X=O))
BDBM81583(AppppA analog, 12 (X=O))
 BDBM81589(AppppA analog, 15 (X=O))
BDBM81589(AppppA analog, 15 (X=O))
 BDBM50056973(CHEMBL519155 | NSC-255523)
BDBM50056973(CHEMBL519155 | NSC-255523)
 BDBM50056973(CHEMBL519155 | NSC-255523)
BDBM50056973(CHEMBL519155 | NSC-255523)
 BDBM50552885(CHEBI:16771 | CHEMBL583986)
BDBM50552885(CHEBI:16771 | CHEMBL583986)
 BDBM81585(AppppA analog, 13 (X=O))
BDBM81585(AppppA analog, 13 (X=O))
 BDBM81577(AppppA analog, 6 (X=O))
BDBM81577(AppppA analog, 6 (X=O))
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
Endoplasmin(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
 BDBM50552885(CHEBI:16771 | CHEMBL583986)
BDBM50552885(CHEBI:16771 | CHEMBL583986)
 BDBM50248004(CHEBI:63599 | Fludara | Fludarabine | Fludarabine ...)
BDBM50248004(CHEBI:63599 | Fludara | Fludarabine | Fludarabine ...)Ki: >1.00E+4nMAssay Description:Inhibition of recombinant human carbonic anhydrase 1 preincubated with enzyme for 15 mins by phenol red dye based stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
 BDBM50248004(CHEBI:63599 | Fludara | Fludarabine | Fludarabine ...)
BDBM50248004(CHEBI:63599 | Fludara | Fludarabine | Fludarabine ...)Ki: >1.00E+4nMAssay Description:Inhibition of recombinant human carbonic anhydrase 2 preincubated with enzyme for 15 mins by phenol red dye based stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
 BDBM86481(AP2A | CAS_0 | NSC_0)
BDBM86481(AP2A | CAS_0 | NSC_0)
 BDBM86481(AP2A | CAS_0 | NSC_0)
BDBM86481(AP2A | CAS_0 | NSC_0)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
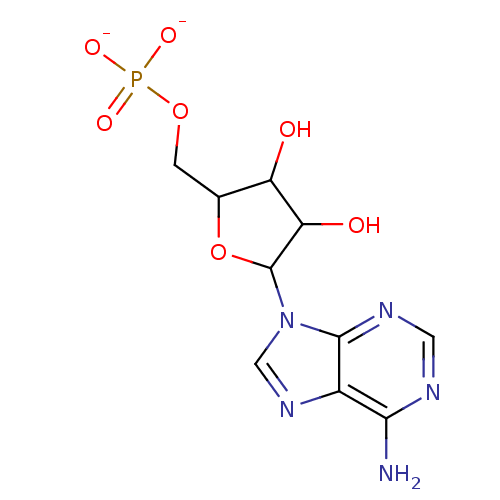 BDBM92538(AMP | [5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2...)
BDBM92538(AMP | [5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)Ki: 8.00E+4nM ΔG°: -23.4kJ/mole IC50: 8.00E+5nMpH: 7.5 T: 2°CAssay Description:Aminoacyl-tRNA synthetase assays were measuring the incorporation of [14C] amino acid into tRNA.More data for this Ligand-Target Pair
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
 BDBM11941(2,5-ADP | ADENOSINE-2 -5 -DIPHOSPHATE | {[(2R,3R,4...)
BDBM11941(2,5-ADP | ADENOSINE-2 -5 -DIPHOSPHATE | {[(2R,3R,4...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
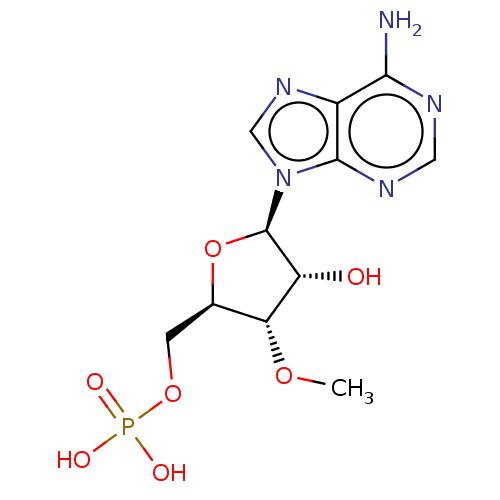 BDBM50027451(CHEMBL3143591 | Phosphoric acid mono-[5-(6-amino-p...)
BDBM50027451(CHEMBL3143591 | Phosphoric acid mono-[5-(6-amino-p...)
 BDBM50027451(CHEMBL3143591 | Phosphoric acid mono-[5-(6-amino-p...)
BDBM50027451(CHEMBL3143591 | Phosphoric acid mono-[5-(6-amino-p...)
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
 BDBM50027451(CHEMBL3143591 | Phosphoric acid mono-[5-(6-amino-p...)
BDBM50027451(CHEMBL3143591 | Phosphoric acid mono-[5-(6-amino-p...)
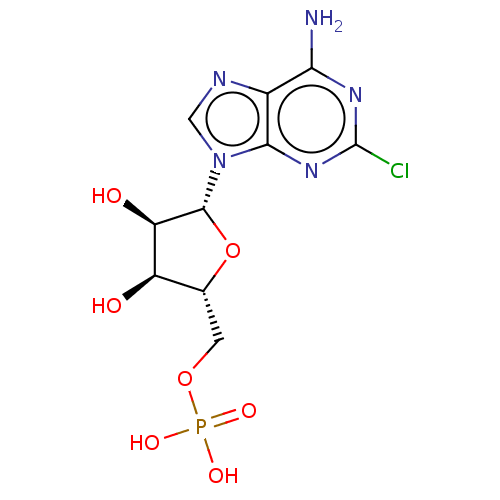 BDBM50367107(CHEMBL3244493 | CHEMBL610643)
BDBM50367107(CHEMBL3244493 | CHEMBL610643)
Ki: 3.20E+6nMAssay Description:Inhibitory activity against rat adenylate kinase II was determined in the presence of AMP, non competitive inhibitionMore data for this Ligand-Target Pair
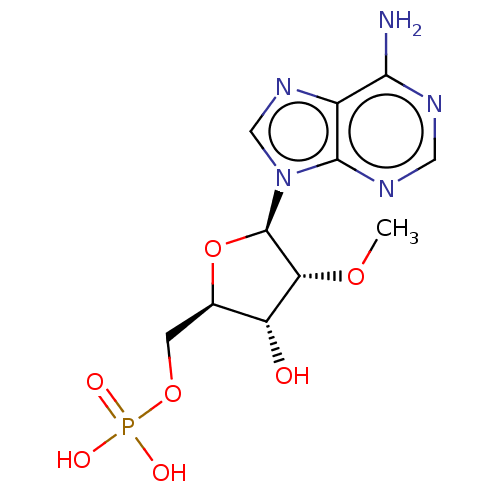 BDBM50027447(CHEMBL1229561 | Phosphoric acid mono-[5-(6-amino-p...)
BDBM50027447(CHEMBL1229561 | Phosphoric acid mono-[5-(6-amino-p...)
 BDBM50027447(CHEMBL1229561 | Phosphoric acid mono-[5-(6-amino-p...)
BDBM50027447(CHEMBL1229561 | Phosphoric acid mono-[5-(6-amino-p...)
 BDBM50367106(CHEMBL1230507 | CHEMBL609083)
BDBM50367106(CHEMBL1230507 | CHEMBL609083)
Ki: 4.50E+6nMAssay Description:Inhibitory activity against rat Adenylate kinase M isoenzyme was determined in the presence of ATP, non competitive inhibitionMore data for this Ligand-Target Pair
 BDBM50027447(CHEMBL1229561 | Phosphoric acid mono-[5-(6-amino-p...)
BDBM50027447(CHEMBL1229561 | Phosphoric acid mono-[5-(6-amino-p...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
Ki: 9.80E+6nMAssay Description:Inhibitory activity against rat adenylate kinase II was determined in the presence of AMP, non competitive inhibitionMore data for this Ligand-Target Pair
Ki: 1.60E+7nMAssay Description:Inhibitory activity against rat Adenylate kinase M isoenzyme was determined in the presence of ATP, non competitive inhibitionMore data for this Ligand-Target Pair
IC50: 0.950nMAssay Description:Inhibitory activity to prevent binding of added ppp5'A2'p5'A2'pA2'p5'A3'[32P]p5' (c3 label) to RNase L in mouse L cellsMore data for this Ligand-Target Pair
IC50: 1.10nMAssay Description:Inhibitory activity to prevent binding of added ppp5'A2'p5'A2'pA2'p5'A3'[32P]p5' (c3 label) to RNase L in human Daudi lymphoblastoid cellsMore data for this Ligand-Target Pair
 BDBM50085554(5'-O-MONOPHOSPHORYLADENYLYL(2'->5')ADENYLYL(2'->5'...)
BDBM50085554(5'-O-MONOPHOSPHORYLADENYLYL(2'->5')ADENYLYL(2'->5'...)
IC50: 20nMAssay Description:Inhibitory activity to prevent binding of added ppp5'A2'p5'A2'pA2'p5'A3'[32P]p5' (c3 label) to RNase L in rabbit reticulocytesMore data for this Ligand-Target Pair
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM50318030(((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-(methylamino)-9H...)
BDBM50318030(((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-(methylamino)-9H...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
P2Y purinoceptor 1(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
IC50: 210nMAssay Description:Inhibition concentration required against wild type strain P2Y1 receptor expressed in COS-7 cells is determined using [3H]-MRS2279 as radioligandMore data for this Ligand-Target Pair
 BDBM50318030(((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-(methylamino)-9H...)
BDBM50318030(((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-(methylamino)-9H...)
 BDBM50013703((ADP)[5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetra...)
BDBM50013703((ADP)[5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetra...)
P2Y purinoceptor 1(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
 BDBM50062291(CHEMBL54116 | Phosphoric acid mono-[2-(6-methylami...)
BDBM50062291(CHEMBL54116 | Phosphoric acid mono-[2-(6-methylami...)IC50: 324nMAssay Description:Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATPMore data for this Ligand-Target Pair
P2Y purinoceptor 1(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
IC50: 330nMAssay Description:Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATPMore data for this Ligand-Target Pair
P2Y purinoceptor 1(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
P2Y purinoceptor 1(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
 BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)
BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)
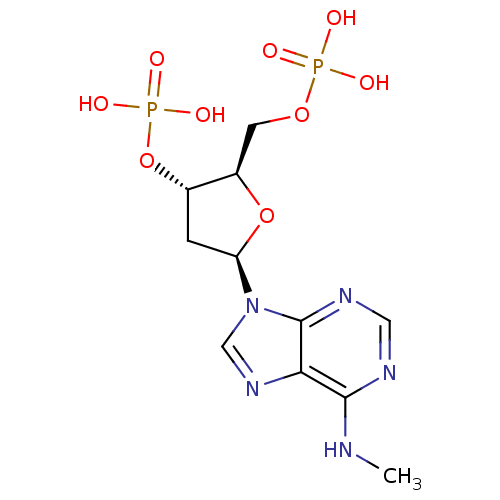 BDBM50318029(CHEMBL1096400 | diammonium (2R,3S,5R)-2-[(hydrogen...)
BDBM50318029(CHEMBL1096400 | diammonium (2R,3S,5R)-2-[(hydrogen...)IC50: 508nMAssay Description:Antagonist activity at human GPR17 expressed in human 1321N1 cells assessed as inhibition of UDP-glucose-induced [35S]GTPgammaS binding after 30 mins...More data for this Ligand-Target Pair
P2Y purinoceptor 1(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
 BDBM50318029(CHEMBL1096400 | diammonium (2R,3S,5R)-2-[(hydrogen...)
BDBM50318029(CHEMBL1096400 | diammonium (2R,3S,5R)-2-[(hydrogen...)P2Y purinoceptor 1(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
P2Y purinoceptor 1(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
P2Y purinoceptor 1(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
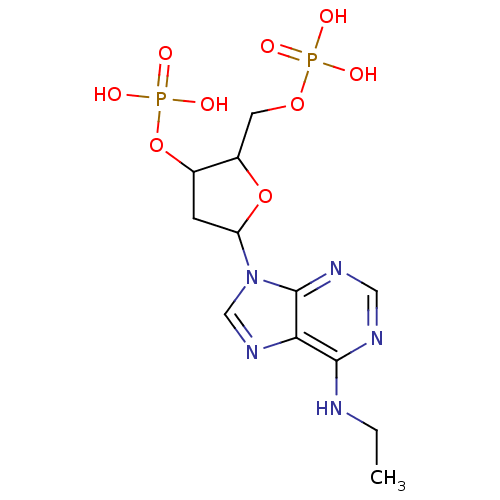 BDBM50062276(CHEMBL55247 | Phosphoric acid mono-[5-(6-ethylamin...)
BDBM50062276(CHEMBL55247 | Phosphoric acid mono-[5-(6-ethylamin...)IC50: 1.08E+3nMAssay Description:Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATPMore data for this Ligand-Target Pair
 BDBM50318030(((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-(methylamino)-9H...)
BDBM50318030(((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-(methylamino)-9H...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
P2Y purinoceptor 1(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)IC50: 4.19E+3nMAssay Description:Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATPMore data for this Ligand-Target Pair
 BDBM50062287(CHEMBL298487 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062287(CHEMBL298487 | Phosphoric acid mono-[5-(6-amino-pu...)
 BDBM50062287(CHEMBL298487 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062287(CHEMBL298487 | Phosphoric acid mono-[5-(6-amino-pu...)IC50: 5.76E+3nMAssay Description:Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATPMore data for this Ligand-Target Pair
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
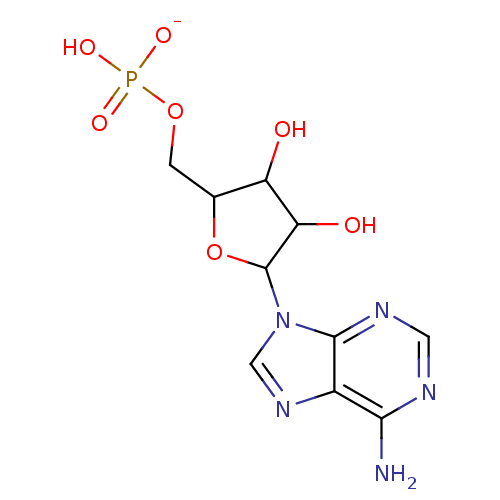 BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)
BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)
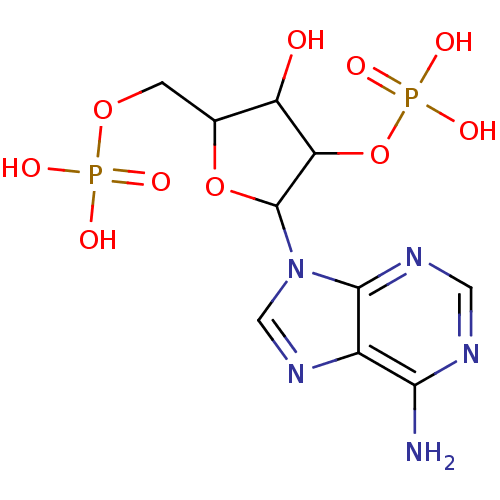 BDBM50062282(CHEMBL58764 | Phosphoric acid mono-[2-(6-amino-pur...)
BDBM50062282(CHEMBL58764 | Phosphoric acid mono-[2-(6-amino-pur...)IC50: 8.46E+3nMAssay Description:Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATPMore data for this Ligand-Target Pair
 BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)
BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
 BDBM50062285(CHEMBL292442 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062285(CHEMBL292442 | Phosphoric acid mono-[5-(6-amino-pu...)IC50: 1.24E+4nMAssay Description:Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATPMore data for this Ligand-Target Pair
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)IC50: 1.32E+4nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute Ass...More data for this Ligand-Target Pair
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
 BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)
BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)
IC50: 4.00E+4nMAssay Description:Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assayMore data for this Ligand-Target Pair
 BDBM50062281(CHEMBL59090 | Phosphoric acid mono-[5-(6-dimethyla...)
BDBM50062281(CHEMBL59090 | Phosphoric acid mono-[5-(6-dimethyla...)IC50: 4.67E+4nMAssay Description:Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATPMore data for this Ligand-Target Pair
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)IC50: 7.00E+4nMAssay Description:Inhibition of human TRPM2 assessed as reduction in ADPR-induced channel currents by whole cell patch clamp electrophysiology methodMore data for this Ligand-Target Pair
 BDBM50318030(((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-(methylamino)-9H...)
BDBM50318030(((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-(methylamino)-9H...)
 BDBM50318030(((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-(methylamino)-9H...)
BDBM50318030(((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-(methylamino)-9H...)
 BDBM50318030(((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-(methylamino)-9H...)
BDBM50318030(((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-(methylamino)-9H...)
 BDBM50318030(((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-(methylamino)-9H...)
BDBM50318030(((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-(methylamino)-9H...)
 BDBM50318030(((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-(methylamino)-9H...)
BDBM50318030(((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-(methylamino)-9H...)
 BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)
BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)IC50: 1.22E+5nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute Ass...More data for this Ligand-Target Pair
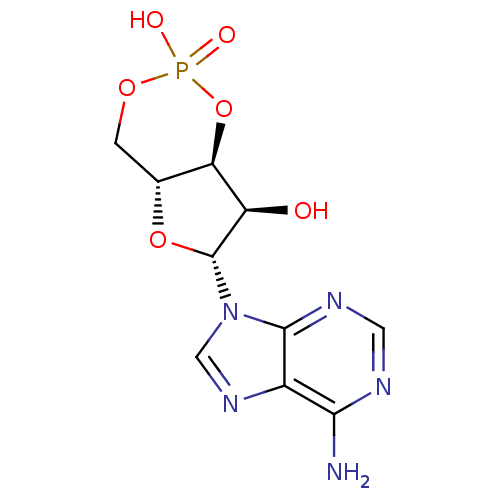 BDBM10851((4aR,6R,7R,7aS)-6-(6-amino-9H-purin-9-yl)-2,7-dihy...)
BDBM10851((4aR,6R,7R,7aS)-6-(6-amino-9H-purin-9-yl)-2,7-dihy...)
 BDBM50248004(CHEBI:63599 | Fludara | Fludarabine | Fludarabine ...)
BDBM50248004(CHEBI:63599 | Fludara | Fludarabine | Fludarabine ...)
 BDBM50248004(CHEBI:63599 | Fludara | Fludarabine | Fludarabine ...)
BDBM50248004(CHEBI:63599 | Fludara | Fludarabine | Fludarabine ...)
 BDBM50248004(CHEBI:63599 | Fludara | Fludarabine | Fludarabine ...)
BDBM50248004(CHEBI:63599 | Fludara | Fludarabine | Fludarabine ...)
 BDBM50248004(CHEBI:63599 | Fludara | Fludarabine | Fludarabine ...)
BDBM50248004(CHEBI:63599 | Fludara | Fludarabine | Fludarabine ...)
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
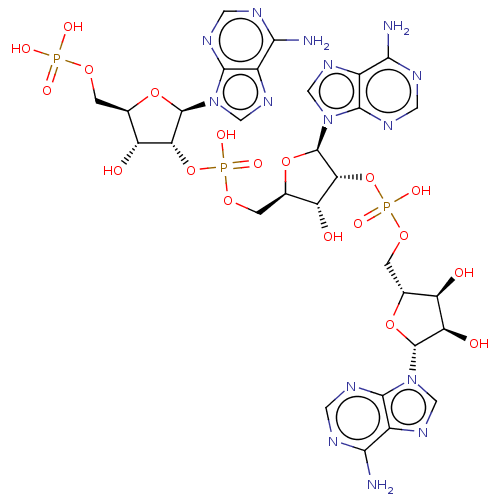 BDBM50024999(CHEMBL414948 | Sulfuric acid mono-{5-(6-amino-puri...)
BDBM50024999(CHEMBL414948 | Sulfuric acid mono-{5-(6-amino-puri...)IC50: 2.00E+5nMAssay Description:Compound was tested for activation of RNase L by measuring concentration required for 50% inhibition of protein synthesis in mouse L cell extractsMore data for this Ligand-Target Pair
 BDBM50192453(AMP(2-) | Adenosine-5-monophosphate | LDHA Inhibit...)
BDBM50192453(AMP(2-) | Adenosine-5-monophosphate | LDHA Inhibit...)
 BDBM11940(2-AMP | 2-Adenylic acid | Adenosine 2-monophosphat...)
BDBM11940(2-AMP | 2-Adenylic acid | Adenosine 2-monophosphat...)
 BDBM50280244(Phosphoric acid mono-[(2R,3R,4S,5R)-5-(6-amino-pur...)
BDBM50280244(Phosphoric acid mono-[(2R,3R,4S,5R)-5-(6-amino-pur...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
P2Y purinoceptor 1(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM11941(2,5-ADP | ADENOSINE-2 -5 -DIPHOSPHATE | {[(2R,3R,4...)
BDBM11941(2,5-ADP | ADENOSINE-2 -5 -DIPHOSPHATE | {[(2R,3R,4...)
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
P2Y purinoceptor 6(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)EC50: 6.50E+4nMAssay Description:Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP productionMore data for this Ligand-Target Pair
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
P2Y purinoceptor 1(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)EC50: 1.73E+4nMAssay Description:Measure of Agonist Potency at human P2Y purinoceptor 11 (hP2Y11) stably expressed in 131N1 astrocytoma cell at 10 uMMore data for this Ligand-Target Pair
P2Y purinoceptor 1(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)P2Y purinoceptor 1(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
P2Y purinoceptor 1(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
 BDBM50085554(5'-O-MONOPHOSPHORYLADENYLYL(2'->5')ADENYLYL(2'->5'...)
BDBM50085554(5'-O-MONOPHOSPHORYLADENYLYL(2'->5')ADENYLYL(2'->5'...)EC50: 0.150nMAssay Description:Activation of purified recombinant human Ribonuclease L by the compound was measured as degradation of poly (U) 3'[32P]p5'C3'pMore data for this Ligand-Target Pair
 BDBM50152834(2''-5--oligoadenylate derivative | [(2R,3R,4R,5R)-...)
BDBM50152834(2''-5--oligoadenylate derivative | [(2R,3R,4R,5R)-...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)Kd: 4.10E+3nMAssay Description:Binding affinity to recombinant human biotinylated N-terminal GST-tagged autophosphorylated TAK1 (1 to 303 residues) fused with TAB1 (437 to 504 resi...More data for this Ligand-Target Pair
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)Kd: 3.70E+5nMAssay Description:Binding affinity to recombinant human biotinylated N-terminal GST-tagged non-autophosphorylated TAK1 (1 to 303 residues) fused with TAB1 (437 to 504 ...More data for this Ligand-Target Pair
 BDBM50509429(CHEBI:71578 | CHEMBL1229884)
BDBM50509429(CHEBI:71578 | CHEMBL1229884)
 BDBM50509429(CHEBI:71578 | CHEMBL1229884)
BDBM50509429(CHEBI:71578 | CHEMBL1229884)
 BDBM50509429(CHEBI:71578 | CHEMBL1229884)
BDBM50509429(CHEBI:71578 | CHEMBL1229884)
 BDBM50509429(CHEBI:71578 | CHEMBL1229884)
BDBM50509429(CHEBI:71578 | CHEMBL1229884)
 BDBM50509429(CHEBI:71578 | CHEMBL1229884)
BDBM50509429(CHEBI:71578 | CHEMBL1229884)
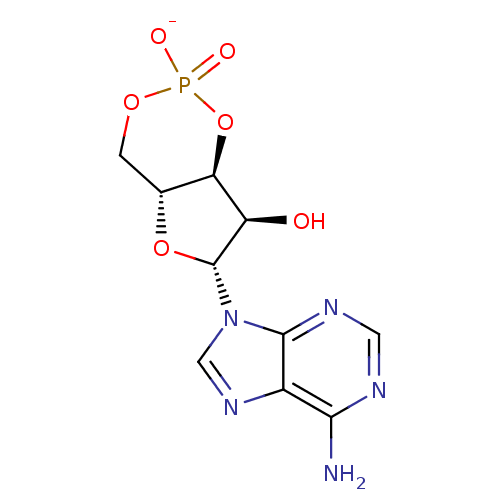 BDBM81289(Adenosine 3',5'-cyclic monophosphate sodiu...)
BDBM81289(Adenosine 3',5'-cyclic monophosphate sodiu...)
 BDBM81289(Adenosine 3',5'-cyclic monophosphate sodiu...)
BDBM81289(Adenosine 3',5'-cyclic monophosphate sodiu...)
 BDBM81289(Adenosine 3',5'-cyclic monophosphate sodiu...)
BDBM81289(Adenosine 3',5'-cyclic monophosphate sodiu...)EC50: 3.87E+3nMAssay Description:Inhibition of 8Fluo-cAMP binding to DEP domain deficient mouse EPAC2 (280 to 988 residues) measured after 5 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
 BDBM81289(Adenosine 3',5'-cyclic monophosphate sodiu...)
BDBM81289(Adenosine 3',5'-cyclic monophosphate sodiu...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
Endoplasmin(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
 BDBM50552885(CHEBI:16771 | CHEMBL583986)
BDBM50552885(CHEBI:16771 | CHEMBL583986)
 BDBM50552885(CHEBI:16771 | CHEMBL583986)
BDBM50552885(CHEBI:16771 | CHEMBL583986)
 BDBM525923(US11185100, TABLE 6.1)
BDBM525923(US11185100, TABLE 6.1)
 BDBM525923(US11185100, TABLE 6.1)
BDBM525923(US11185100, TABLE 6.1)
 BDBM525952(US11185100, TABLE 6.5)
BDBM525952(US11185100, TABLE 6.5)EC50: >1.00E+6nMAssay Description:HEK293 cells that stably express T1R3 and inducibly express T1R1 were exposed to nucleotide derivatives alone to activate the umami receptor. Activat...More data for this Ligand-Target Pair
 BDBM525952(US11185100, TABLE 6.5)
BDBM525952(US11185100, TABLE 6.5)
 BDBM525952(US11185100, TABLE 6.5)
BDBM525952(US11185100, TABLE 6.5)
 BDBM525952(US11185100, TABLE 6.5)
BDBM525952(US11185100, TABLE 6.5)
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)EC50: >1.00E+4nMAssay Description:HEK293 cells that stably express T1R3 and inducibly express T1R1 were exposed to nucleotide derivatives alone to activate the umami receptor. Activat...More data for this Ligand-Target Pair
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)EC50: >3.00E+4nMAssay Description:HEK293 cells that stably express T1R3 and inducibly express T1R1 were exposed to nucleotide derivatives alone to activate the umami receptor. Activat...More data for this Ligand-Target Pair
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)EC50: >3.00E+4nMAssay Description:HEK293 cells that stably express T1R3 and inducibly express T1R1 were exposed to nucleotide derivatives alone to activate the umami receptor. Activat...More data for this Ligand-Target Pair
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
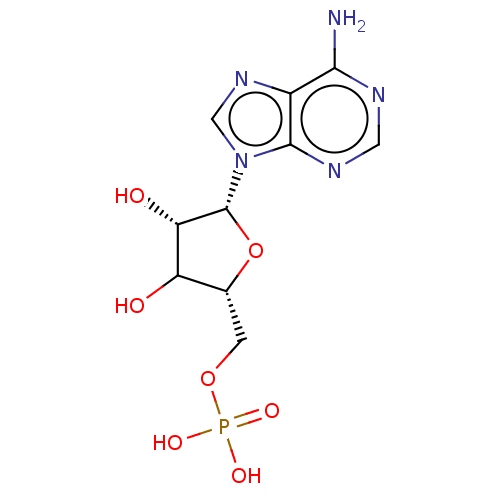 BDBM526098(US11185100, TABLE 7.2)
BDBM526098(US11185100, TABLE 7.2)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM526139(US11185100, TABLE 7.5)
BDBM526139(US11185100, TABLE 7.5)
 BDBM526148(US11185100, TABLE 7.6)
BDBM526148(US11185100, TABLE 7.6)
 BDBM526200(US11185100, TABLE 7.8)
BDBM526200(US11185100, TABLE 7.8)
 BDBM526234(US11185100, TABLE 7.14)
BDBM526234(US11185100, TABLE 7.14)
 BDBM50509429(CHEBI:71578 | CHEMBL1229884)
BDBM50509429(CHEBI:71578 | CHEMBL1229884)
 BDBM50509429(CHEBI:71578 | CHEMBL1229884)
BDBM50509429(CHEBI:71578 | CHEMBL1229884)
 BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)
BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)
 BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)
BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)EC50: >3.00E+5nMAssay Description:Keywords: GSK3beta, dose response, kinase, inhibition, HTS Assay Overview: The glycogen synthase kinase-3 beta (GSK-3b) is a known master regulator f...More data for this Ligand-Target Pair
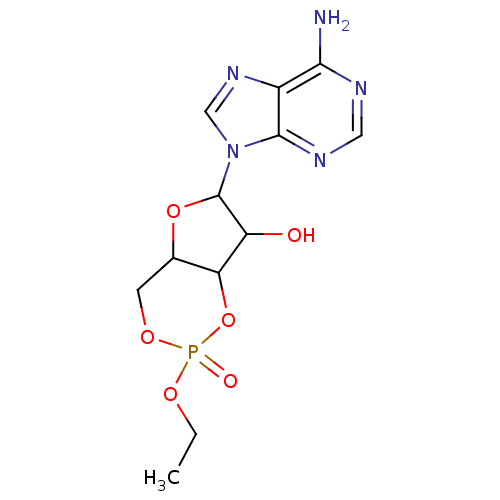 BDBM81287(6-(6-aminopurin-9-yl)-2-ethoxy-2-oxidanylidene-4a,...)
BDBM81287(6-(6-aminopurin-9-yl)-2-ethoxy-2-oxidanylidene-4a,...)
 BDBM81289(Adenosine 3',5'-cyclic monophosphate sodiu...)
BDBM81289(Adenosine 3',5'-cyclic monophosphate sodiu...)EC50: 68.0nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Affiliation: The Scripps Research Institute, TSRI Assa...More data for this Ligand-Target Pair
 BDBM81287(6-(6-aminopurin-9-yl)-2-ethoxy-2-oxidanylidene-4a,...)
BDBM81287(6-(6-aminopurin-9-yl)-2-ethoxy-2-oxidanylidene-4a,...)
 BDBM81289(Adenosine 3',5'-cyclic monophosphate sodiu...)
BDBM81289(Adenosine 3',5'-cyclic monophosphate sodiu...)EC50: 88.5nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Affiliation: The Scripps Research Institute, TSRI Assa...More data for this Ligand-Target Pair
 BDBM81287(6-(6-aminopurin-9-yl)-2-ethoxy-2-oxidanylidene-4a,...)
BDBM81287(6-(6-aminopurin-9-yl)-2-ethoxy-2-oxidanylidene-4a,...)
 BDBM81289(Adenosine 3',5'-cyclic monophosphate sodiu...)
BDBM81289(Adenosine 3',5'-cyclic monophosphate sodiu...)EC50: 88.5nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Affiliation: The Scripps Research Institute, TSRI Assa...More data for this Ligand-Target Pair
 BDBM81287(6-(6-aminopurin-9-yl)-2-ethoxy-2-oxidanylidene-4a,...)
BDBM81287(6-(6-aminopurin-9-yl)-2-ethoxy-2-oxidanylidene-4a,...)
 BDBM81289(Adenosine 3',5'-cyclic monophosphate sodiu...)
BDBM81289(Adenosine 3',5'-cyclic monophosphate sodiu...)EC50: 68.0nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Affiliation: The Scripps Research Institute, TSRI Assa...More data for this Ligand-Target Pair
 BDBM82124(adenosine-derived inhibitor (Grp78), 1)
BDBM82124(adenosine-derived inhibitor (Grp78), 1)
 BDBM82124(adenosine-derived inhibitor (Grp78), 1)
BDBM82124(adenosine-derived inhibitor (Grp78), 1)Kd: 500nMpH: 7.4 T: 2°CAssay Description:SPR measurements wereperformed on BIAcore T100 instrument (BIAcore GE Healthcare), at25 C on series S NTA chips (certified) according to provider'...More data for this Ligand-Target Pair
 BDBM50192453(AMP(2-) | Adenosine-5-monophosphate | LDHA Inhibit...)
BDBM50192453(AMP(2-) | Adenosine-5-monophosphate | LDHA Inhibit...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM50062282(CHEMBL58764 | Phosphoric acid mono-[2-(6-amino-pur...)
BDBM50062282(CHEMBL58764 | Phosphoric acid mono-[2-(6-amino-pur...)EC50: 1.37E+3nMAssay Description:Agonist activity at P2Y1 receptor measured as capacity to stimulate 50% phospholipase C in turkey erythrocyte membranesMore data for this Ligand-Target Pair
 BDBM50062285(CHEMBL292442 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062285(CHEMBL292442 | Phosphoric acid mono-[5-(6-amino-pu...)EC50: 1.29E+4nMAssay Description:Agonist activity at P2Y1 receptor measured as capacity to stimulate 50% phospholipase C in turkey erythrocyte membranesMore data for this Ligand-Target Pair
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)EC50: 1.28E+3nMAssay Description:Agonist activity at P2Y1 receptor measured as capacity to stimulate 50% phospholipase C in turkey erythrocyte membranesMore data for this Ligand-Target Pair
 BDBM50062287(CHEMBL298487 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062287(CHEMBL298487 | Phosphoric acid mono-[5-(6-amino-pu...)EC50: 6.26E+3nMAssay Description:Agonist activity at P2Y1 receptor measured as capacity to stimulate 50% phospholipase C in turkey erythrocyte membranesMore data for this Ligand-Target Pair
 BDBM50085554(5'-O-MONOPHOSPHORYLADENYLYL(2'->5')ADENYLYL(2'->5'...)
BDBM50085554(5'-O-MONOPHOSPHORYLADENYLYL(2'->5')ADENYLYL(2'->5'...)EC50: 0.200nMAssay Description:Activation of purified recombinant human Ribonuclease L by the compound was measured as degradation of [32P]-pC11U2C7More data for this Ligand-Target Pair
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
 BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)
BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)
 BDBM50189858(CHEMBL434553 | {[(2R,3R,4R,5R)-5-(6-amino-9H-purin...)
BDBM50189858(CHEMBL434553 | {[(2R,3R,4R,5R)-5-(6-amino-9H-purin...)
 BDBM50189859(CHEMBL427600 | {[(2R,3R,4R,5R)-5-(6-amino-9H-purin...)
BDBM50189859(CHEMBL427600 | {[(2R,3R,4R,5R)-5-(6-amino-9H-purin...)
 BDBM50189860(CHEMBL261902 | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin...)
BDBM50189860(CHEMBL261902 | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin...)
 BDBM50189861(CHEMBL424746 | {[(2R,3R,4R,5R)-5-(6-amino-9H-purin...)
BDBM50189861(CHEMBL424746 | {[(2R,3R,4R,5R)-5-(6-amino-9H-purin...)
 BDBM50189863(CHEMBL439290 | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin...)
BDBM50189863(CHEMBL439290 | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin...)
 BDBM50189862(CHEMBL414569 | {[(2R,3R,4R,5R)-5-(6-amino-9H-purin...)
BDBM50189862(CHEMBL414569 | {[(2R,3R,4R,5R)-5-(6-amino-9H-purin...)
 BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)
BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
P2Y purinoceptor 1(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
P2Y purinoceptor 1(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
 BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)
BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)EC50: 14nMAssay Description:Agonist activity at G-protein coupled P2Y1 receptor expressed in human 1321N1 cells assessed as increase in calcium by Fura2 assayMore data for this Ligand-Target Pair
 BDBM50152834(2''-5--oligoadenylate derivative | [(2R,3R,4R,5R)-...)
BDBM50152834(2''-5--oligoadenylate derivative | [(2R,3R,4R,5R)-...)EC50: 9.70nMAssay Description:Inhibition of human RNaseL ANK domain expressed in Escherichia coli assessed as 5' flurescein-r(C11U2C7)-3' RNA cleavageMore data for this Ligand-Target Pair
 BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)
BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)
 BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)
BDBM31995(ADP | Adenosine Diphosphate (ADP) | CHEMBL14830)
 BDBM50062287(CHEMBL298487 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062287(CHEMBL298487 | Phosphoric acid mono-[5-(6-amino-pu...)EC50: 6.29E+3nMAssay Description:Concentration at which 50% of the maximal effect (stimulation of PLC at P2Y1 receptor in the turkey erythrocyte membranes) is reachedMore data for this Ligand-Target Pair
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)EC50: 2.34E+3nMAssay Description:Concentration at which 50% of the maximal effect (stimulation of PLC at P2Y1 receptor in the turkey erythrocyte membranes) is reachedMore data for this Ligand-Target Pair
P2Y purinoceptor 1(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
EC50: 1.30E+4nMAssay Description:The compound was evaluated for antagonist activity against recombinant rat P2X purinoceptor 3 (P2X3) at 30 uM,expressed in Xenopus oocytesMore data for this Ligand-Target Pair
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
EC50: >1.00E+4nMAssay Description:The compound was evaluated for antagonist activity against recombinant rat receptor P2X purinoceptor 2 (P2X2) at 10 uM, expressed in Xenopus oocytesMore data for this Ligand-Target Pair
P2Y purinoceptor 6(Homo sapiens (Human))National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
EC50: >1.00E+5nMAssay Description:The compound was evaluated for agonist activity against phospholipase C coupled recombinant human P2Y purinoceptor 6 (P2Y6)More data for this Ligand-Target Pair
EC50: >1.00E+5nMAssay Description:The compound was evaluated for antagonist activity against phospholipase C coupled recombinant human P2Y purinoceptor 4 (P2Y4)More data for this Ligand-Target Pair
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)EC50: >1.00E+5nMAssay Description:The compound was evaluated for antagonist activity against recombinant rat P2X purinoceptor 3 (P2X3) at 10 uM, expressed in Xenopus oocytesMore data for this Ligand-Target Pair
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
 BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
BDBM50368125(ADENOSINE DIPHOSPHATE | ADP)
EC50: 100nMAssay Description:Agonist activity at human A1AR expressed in HEK293T/17 cells assessed as inhibition of isoproterenol-induced cAMP accumulation incubated for 10 mins ...More data for this Ligand-Target Pair
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)EC50: 500nMAssay Description:Agonist activity at human A1AR expressed in HEK293T/17 cells assessed as inhibition of isoproterenol-induced cAMP accumulation incubated for 10 mins ...More data for this Ligand-Target Pair
EC50: 440nMAssay Description:Agonist activity at human A1AR expressed in HEK293T/17 cells assessed as inhibition of isoproterenol-induced cAMP accumulation incubated for 10 mins ...More data for this Ligand-Target Pair
 BDBM11940(2-AMP | 2-Adenylic acid | Adenosine 2-monophosphat...)
BDBM11940(2-AMP | 2-Adenylic acid | Adenosine 2-monophosphat...)EC50: 490nMAssay Description:Agonist activity at human A1AR expressed in HEK293T/17 cells assessed as inhibition of isoproterenol-induced cAMP accumulation incubated for 10 mins ...More data for this Ligand-Target Pair
Displayed 1 to 50 (of 286 total ) | Next | Last >>