Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB (change energy unit to kcal/mol)
 Found 106 hits in this display
Found 106 hits in this display
 BDBM525149(US10988505, Comparative #1)
BDBM525149(US10988505, Comparative #1)
Prothrombin(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
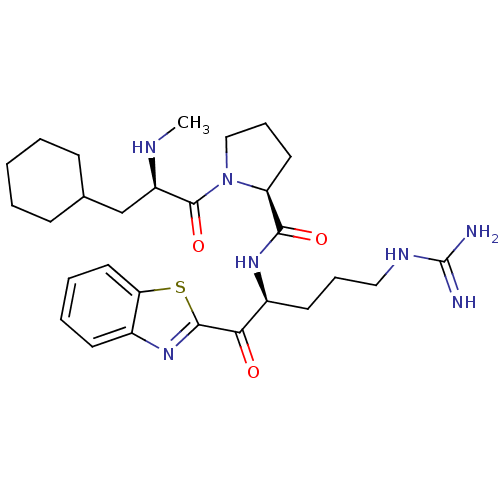 BDBM50139746((S)-1-((R)-3-Cyclohexyl-2-methylamino-propionyl)-p...)
BDBM50139746((S)-1-((R)-3-Cyclohexyl-2-methylamino-propionyl)-p...)
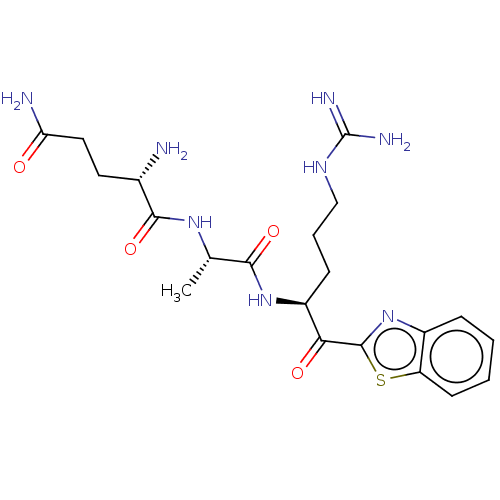 BDBM236493(US10988505, Comparative #2 | US9365853, 4)
BDBM236493(US10988505, Comparative #2 | US9365853, 4)Ki: 0.0880nM ΔG°: -57.4kJ/molepH: 7.4 T: 2°CAssay Description:Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti...More data for this Ligand-Target Pair
 BDBM236493(US10988505, Comparative #2 | US9365853, 4)
BDBM236493(US10988505, Comparative #2 | US9365853, 4)Ki: 0.0880nMAssay Description:Enzymatic assays and Ki determination were performed at room temperature in an assay buffer containing 50 mM Tris-HCl, 150 mM NaCl and 500 μg/mL...More data for this Ligand-Target Pair
Ki: 1.40nM ΔG°: -50.5kJ/molepH: 7.4 T: 2°CAssay Description:Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti...More data for this Ligand-Target Pair
 BDBM50131980(1-Acetyl-pyrrolidine-2-carboxylic acid [1-(benzoth...)
BDBM50131980(1-Acetyl-pyrrolidine-2-carboxylic acid [1-(benzoth...)
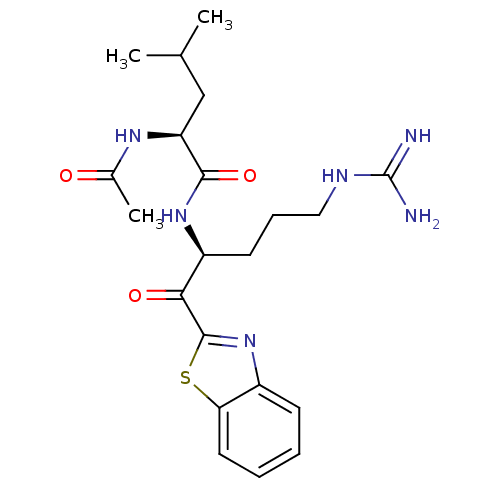 BDBM50131982(2-Acetylamino-4-methyl-pentanoic acid [1-(benzothi...)
BDBM50131982(2-Acetylamino-4-methyl-pentanoic acid [1-(benzothi...)
Ki: 9.5nM ΔG°: -45.8kJ/molepH: 7.4 T: 2°CAssay Description:Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti...More data for this Ligand-Target Pair
 BDBM50131983(1-Acetyl-4-oxo-pyrrolidine-2-carboxylic acid [1-(b...)
BDBM50131983(1-Acetyl-4-oxo-pyrrolidine-2-carboxylic acid [1-(b...)
Tryptase beta-2(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
 BDBM50131980(1-Acetyl-pyrrolidine-2-carboxylic acid [1-(benzoth...)
BDBM50131980(1-Acetyl-pyrrolidine-2-carboxylic acid [1-(benzoth...)Tryptase beta-2(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
 BDBM50131984(1-Acetyl-azetidine-2-carboxylic acid [1-(benzothia...)
BDBM50131984(1-Acetyl-azetidine-2-carboxylic acid [1-(benzothia...)
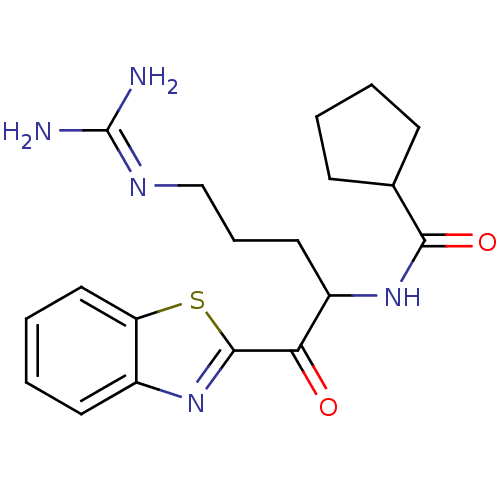 BDBM14083(2-ketobenzothiazole 23 | N-[1-(1,3-benzothiazol-2-...)
BDBM14083(2-ketobenzothiazole 23 | N-[1-(1,3-benzothiazol-2-...)
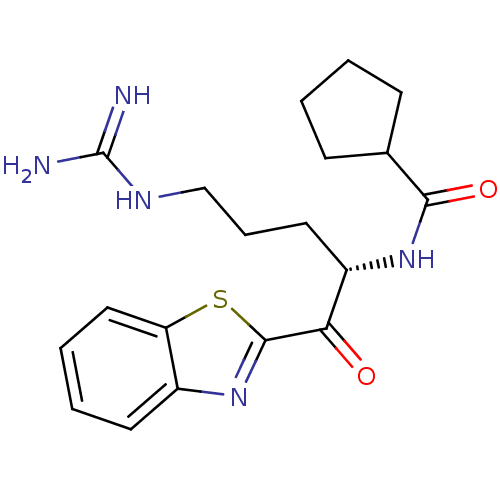 BDBM50139747(CHEMBL164138 | CYCLOPENTANECARBOXYLIC ACID [1-(BEN...)
BDBM50139747(CHEMBL164138 | CYCLOPENTANECARBOXYLIC ACID [1-(BEN...)
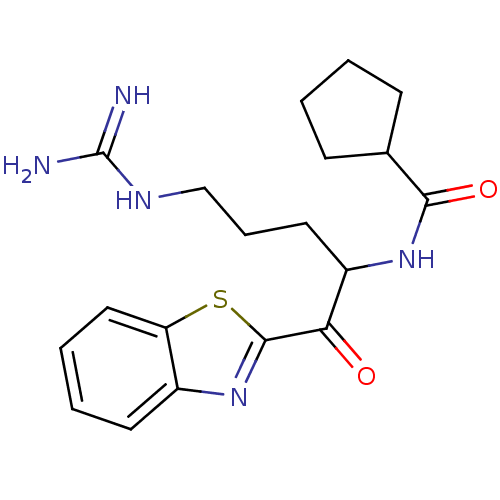 BDBM50131979(CHEMBL340547 | Cyclopentanecarboxylic acid [1-(ben...)
BDBM50131979(CHEMBL340547 | Cyclopentanecarboxylic acid [1-(ben...)
Tryptase beta-2(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
 BDBM50131982(2-Acetylamino-4-methyl-pentanoic acid [1-(benzothi...)
BDBM50131982(2-Acetylamino-4-methyl-pentanoic acid [1-(benzothi...)
 BDBM50131984(1-Acetyl-azetidine-2-carboxylic acid [1-(benzothia...)
BDBM50131984(1-Acetyl-azetidine-2-carboxylic acid [1-(benzothia...)
Tryptase beta-2(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
 BDBM50131983(1-Acetyl-4-oxo-pyrrolidine-2-carboxylic acid [1-(b...)
BDBM50131983(1-Acetyl-4-oxo-pyrrolidine-2-carboxylic acid [1-(b...)Tryptase beta-2(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
 BDBM50131979(CHEMBL340547 | Cyclopentanecarboxylic acid [1-(ben...)
BDBM50131979(CHEMBL340547 | Cyclopentanecarboxylic acid [1-(ben...)
 BDBM50131981(CHEMBL340098 | N-{[1-(Benzothiazole-2-carbonyl)-4-...)
BDBM50131981(CHEMBL340098 | N-{[1-(Benzothiazole-2-carbonyl)-4-...)
Ki: 457nM ΔG°: -36.2kJ/molepH: 7.4 T: 2°CAssay Description:Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti...More data for this Ligand-Target Pair
Tryptase beta-2(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
 BDBM50131981(CHEMBL340098 | N-{[1-(Benzothiazole-2-carbonyl)-4-...)
BDBM50131981(CHEMBL340098 | N-{[1-(Benzothiazole-2-carbonyl)-4-...)
 BDBM50131976(1-Acetyl-pyrrolidine-2-carboxylic acid [1-(benzoth...)
BDBM50131976(1-Acetyl-pyrrolidine-2-carboxylic acid [1-(benzoth...)
Prothrombin(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
 BDBM14083(2-ketobenzothiazole 23 | N-[1-(1,3-benzothiazol-2-...)
BDBM14083(2-ketobenzothiazole 23 | N-[1-(1,3-benzothiazol-2-...)Prothrombin(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
 BDBM50139747(CHEMBL164138 | CYCLOPENTANECARBOXYLIC ACID [1-(BEN...)
BDBM50139747(CHEMBL164138 | CYCLOPENTANECARBOXYLIC ACID [1-(BEN...)Tryptase beta-2(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
 BDBM50131976(1-Acetyl-pyrrolidine-2-carboxylic acid [1-(benzoth...)
BDBM50131976(1-Acetyl-pyrrolidine-2-carboxylic acid [1-(benzoth...)Prothrombin(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
 BDBM14083(2-ketobenzothiazole 23 | N-[1-(1,3-benzothiazol-2-...)
BDBM14083(2-ketobenzothiazole 23 | N-[1-(1,3-benzothiazol-2-...)
IC50: 1.70nMAssay Description:Inhibition of recombinant human C-terminal 10-His tagged hepsin (Arg45 to Leu417) expressed in mouse myeloma cells using Boc-QAR-AMC as substrate pre...More data for this Ligand-Target Pair
Tryptase beta-2(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
 BDBM50131976(1-Acetyl-pyrrolidine-2-carboxylic acid [1-(benzoth...)
BDBM50131976(1-Acetyl-pyrrolidine-2-carboxylic acid [1-(benzoth...)Trypsin-3(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
 BDBM50131981(CHEMBL340098 | N-{[1-(Benzothiazole-2-carbonyl)-4-...)
BDBM50131981(CHEMBL340098 | N-{[1-(Benzothiazole-2-carbonyl)-4-...)
IC50: 2.20nMAssay Description:Inhibition of recombinant human N-terminal met-His6-tagged matriptase catalytic domain (Gly596 to Val855 residues) expressed in Escherichia coli usin...More data for this Ligand-Target Pair
IC50: 2.5nMAssay Description:Inhibition of recombinant human N-terminal met-His6-tagged matriptase catalytic domain (Gly596 to Val855 residues) expressed in Escherichia coli usin...More data for this Ligand-Target Pair
IC50: 3.10nMAssay Description:Inhibition of recombinant human N-terminal met-His6-tagged matriptase catalytic domain (Gly596 to Val855 residues) expressed in Escherichia coli usin...More data for this Ligand-Target Pair
IC50: 3.80nMAssay Description:Inhibition of recombinant human N-terminal met-His6-tagged matriptase catalytic domain (Gly596 to Val855 residues) expressed in Escherichia coli usin...More data for this Ligand-Target Pair
Trypsin-3(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
 BDBM50131976(1-Acetyl-pyrrolidine-2-carboxylic acid [1-(benzoth...)
BDBM50131976(1-Acetyl-pyrrolidine-2-carboxylic acid [1-(benzoth...)
IC50: 4.40nMAssay Description:Inhibition of recombinant human N-terminal met-His6-tagged matriptase catalytic domain (Gly596 to Val855 residues) expressed in Escherichia coli usin...More data for this Ligand-Target Pair
Trypsin-3(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
 BDBM50131984(1-Acetyl-azetidine-2-carboxylic acid [1-(benzothia...)
BDBM50131984(1-Acetyl-azetidine-2-carboxylic acid [1-(benzothia...)
 BDBM397449(US10676470, Compound 24 | US11332464, Compound 24 ...)
BDBM397449(US10676470, Compound 24 | US11332464, Compound 24 ...)
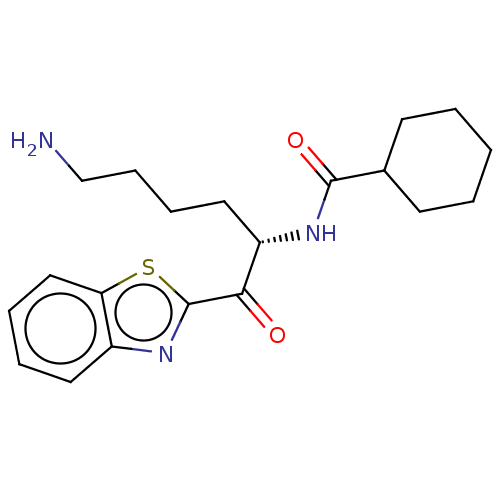 BDBM397433(US10676470, Compound 2 | US11332464, Compound 2 | ...)
BDBM397433(US10676470, Compound 2 | US11332464, Compound 2 | ...)IC50: 5.5nMAssay Description:Buffer: pH=7.5, 100 mM Tris-HCl, 75 mM NaCl, 2.5 mM CaCl2, 10 mM cysteine, 1% DMSO after all additions. Protein: 0.1 nM Kgp, isolated from culture of...More data for this Ligand-Target Pair
 BDBM397456(US10676470, Compound 33 | US11332464, Compound 33 ...)
BDBM397456(US10676470, Compound 33 | US11332464, Compound 33 ...)
 BDBM397449(US10676470, Compound 24 | US11332464, Compound 24 ...)
BDBM397449(US10676470, Compound 24 | US11332464, Compound 24 ...)IC50: 5.5nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
 BDBM397433(US10676470, Compound 2 | US11332464, Compound 2 | ...)
BDBM397433(US10676470, Compound 2 | US11332464, Compound 2 | ...)IC50: 5.5nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
 BDBM397456(US10676470, Compound 33 | US11332464, Compound 33 ...)
BDBM397456(US10676470, Compound 33 | US11332464, Compound 33 ...)
 BDBM397449(US10676470, Compound 24 | US11332464, Compound 24 ...)
BDBM397449(US10676470, Compound 24 | US11332464, Compound 24 ...)IC50: 5.5nMAssay Description:The specific assay conditions were as follows. Buffer: pH=7.5, 100 mM Tris-HCl, 75 mM NaCl, 2.5 mM CaCl2, 10 mM cysteine, 1% DMSO after all additions...More data for this Ligand-Target Pair
 BDBM397433(US10676470, Compound 2 | US11332464, Compound 2 | ...)
BDBM397433(US10676470, Compound 2 | US11332464, Compound 2 | ...)IC50: 5.5nMAssay Description:The specific assay conditions were as follows. Buffer: pH=7.5, 100 mM Tris-HCl, 75 mM NaCl, 2.5 mM CaCl2, 10 mM cysteine, 1% DMSO after all additions...More data for this Ligand-Target Pair
 BDBM397456(US10676470, Compound 33 | US11332464, Compound 33 ...)
BDBM397456(US10676470, Compound 33 | US11332464, Compound 33 ...)IC50: 5.5nMAssay Description:Buffer: pH=7.5, 100 mM Tris-HCl, 75 mM NaCl, 2.5 mM CaCl2, 10 mM cysteine, 1% DMSO after all additions. Protein: 0.1 nM Kgp, isolated from culture of...More data for this Ligand-Target Pair
IC50: 5.90nMAssay Description:Inhibition of recombinant human C-terminal 10-His tagged hepsin (Arg45 to Leu417) expressed in mouse myeloma cells using Boc-QAR-AMC as substrate pre...More data for this Ligand-Target Pair
IC50: 6.30nMAssay Description:Inhibition of recombinant human N-terminal met-His6-tagged matriptase catalytic domain (Gly596 to Val855 residues) expressed in Escherichia coli usin...More data for this Ligand-Target Pair
IC50: 7.70nMAssay Description:Inhibition of recombinant human N-terminal met-His6-tagged matriptase catalytic domain (Gly596 to Val855 residues) expressed in Escherichia coli usin...More data for this Ligand-Target Pair
IC50: 8.40nMAssay Description:Inhibition of recombinant human C-terminal 10-His tagged hepsin (Arg45 to Leu417) expressed in mouse myeloma cells using Boc-QAR-AMC as substrate pre...More data for this Ligand-Target Pair
IC50: 8.80nMAssay Description:Inhibition of recombinant human C-terminal 10-His tagged hepsin (Arg45 to Leu417) expressed in mouse myeloma cells using Boc-QAR-AMC as substrate pre...More data for this Ligand-Target Pair
IC50: 10nMAssay Description:Inhibition of recombinant human C-terminal 10-His tagged hepsin (Arg45 to Leu417) expressed in mouse myeloma cells using Boc-QAR-AMC as substrate pre...More data for this Ligand-Target Pair
IC50: 11nMAssay Description:Inhibition of recombinant human C-terminal 10-His tagged hepsin (Arg45 to Leu417) expressed in mouse myeloma cells using Boc-QAR-AMC as substrate pre...More data for this Ligand-Target Pair
 BDBM397433(US10676470, Compound 2 | US11332464, Compound 2 | ...)
BDBM397433(US10676470, Compound 2 | US11332464, Compound 2 | ...)IC50: 17.5nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
 BDBM397433(US10676470, Compound 2 | US11332464, Compound 2 | ...)
BDBM397433(US10676470, Compound 2 | US11332464, Compound 2 | ...)IC50: 17.5nMAssay Description:The specific assay conditions were as follows. Buffer: pH=7.5, 100 mM Tris-HCl, 75 mM NaCl, 2.5 mM CaCl2, 10 mM cysteine, 1% DMSO after all additions...More data for this Ligand-Target Pair
 BDBM397433(US10676470, Compound 2 | US11332464, Compound 2 | ...)
BDBM397433(US10676470, Compound 2 | US11332464, Compound 2 | ...)IC50: 17.5nMAssay Description:Buffer: pH=7.5, 100 mM Tris-HCl, 75 mM NaCl, 2.5 mM CaCl2, 10 mM cysteine, 1% DMSO after all additions. Protein: 0.1 nM Kgp, isolated from culture of...More data for this Ligand-Target Pair
IC50: 20nMAssay Description:Inhibition of recombinant human N-terminal met-His6-tagged matriptase catalytic domain (Gly596 to Val855 residues) expressed in Escherichia coli usin...More data for this Ligand-Target Pair
IC50: 22nMAssay Description:Inhibition of recombinant human C-terminal 10-His tagged hepsin (Arg45 to Leu417) expressed in mouse myeloma cells using Boc-QAR-AMC as substrate pre...More data for this Ligand-Target Pair
IC50: 25nMAssay Description:Inhibition of recombinant human C-terminal 10-His tagged hepsin (Arg45 to Leu417) expressed in mouse myeloma cells using Boc-QAR-AMC as substrate pre...More data for this Ligand-Target Pair
IC50: 723nMAssay Description:Inhibition of recombinant C-terminal 10His-tagged human coagulation factor 10a (Leu24 to Lys488 residues) expressed in baculovirus infected Sf9 insec...More data for this Ligand-Target Pair
IC50: 1.16E+3nMAssay Description:Inhibition of recombinant C-terminal 10His-tagged human coagulation factor 10a (Leu24 to Lys488 residues) expressed in baculovirus infected Sf9 insec...More data for this Ligand-Target Pair
IC50: 1.71E+3nMAssay Description:Inhibition of recombinant C-terminal 10His-tagged human coagulation factor 10a (Leu24 to Lys488 residues) expressed in baculovirus infected Sf9 insec...More data for this Ligand-Target Pair
IC50: 2.05E+3nMAssay Description:Inhibition of recombinant C-terminal 10His-tagged human coagulation factor 10a (Leu24 to Lys488 residues) expressed in baculovirus infected Sf9 insec...More data for this Ligand-Target Pair
IC50: 4.47E+3nMAssay Description:Inhibition of recombinant human HGFA serine protease domain using Boc-QLR-AMC as substrate preincubated for 30 mins followed by substrate addition by...More data for this Ligand-Target Pair
IC50: 4.65E+3nMAssay Description:Inhibition of recombinant C-terminal 10His-tagged human coagulation factor 10a (Leu24 to Lys488 residues) expressed in baculovirus infected Sf9 insec...More data for this Ligand-Target Pair
IC50: 5.05E+3nMAssay Description:Inhibition of recombinant human HGFA serine protease domain using Boc-QLR-AMC as substrate preincubated for 30 mins followed by substrate addition by...More data for this Ligand-Target Pair
IC50: 6.18E+3nMAssay Description:Inhibition of recombinant C-terminal 10His-tagged human coagulation factor 10a (Leu24 to Lys488 residues) expressed in baculovirus infected Sf9 insec...More data for this Ligand-Target Pair
Prothrombin(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
IC50: 8.61E+3nMAssay Description:Inhibition of recombinant human HGFA serine protease domain using Boc-QLR-AMC as substrate preincubated for 30 mins followed by substrate addition by...More data for this Ligand-Target Pair
IC50: 1.37E+4nMAssay Description:Inhibition of recombinant human HGFA serine protease domain using Boc-QLR-AMC as substrate preincubated for 30 mins followed by substrate addition by...More data for this Ligand-Target Pair
IC50: 1.58E+4nMAssay Description:Inhibition of recombinant human HGFA serine protease domain using Boc-QLR-AMC as substrate preincubated for 30 mins followed by substrate addition by...More data for this Ligand-Target Pair
IC50: 1.61E+4nMAssay Description:Inhibition of recombinant human HGFA serine protease domain using Boc-QLR-AMC as substrate preincubated for 30 mins followed by substrate addition by...More data for this Ligand-Target Pair
Prothrombin(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
IC50: >2.00E+4nMAssay Description:Inhibition of recombinant thrombin (unknown origin) using D-Phe-Pip-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition by c...More data for this Ligand-Target Pair
Prothrombin(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
IC50: >2.00E+4nMAssay Description:Inhibition of recombinant thrombin (unknown origin) using D-Phe-Pip-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition by c...More data for this Ligand-Target Pair
Prothrombin(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
IC50: >2.00E+4nMAssay Description:Inhibition of recombinant thrombin (unknown origin) using D-Phe-Pip-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition by c...More data for this Ligand-Target Pair
Prothrombin(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
IC50: >2.00E+4nMAssay Description:Inhibition of recombinant thrombin (unknown origin) using D-Phe-Pip-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition by c...More data for this Ligand-Target Pair
IC50: >2.00E+4nMAssay Description:Inhibition of recombinant human HGFA serine protease domain using Boc-QLR-AMC as substrate preincubated for 30 mins followed by substrate addition by...More data for this Ligand-Target Pair
IC50: >2.00E+4nMAssay Description:Inhibition of recombinant human HGFA serine protease domain using Boc-QLR-AMC as substrate preincubated for 30 mins followed by substrate addition by...More data for this Ligand-Target Pair
IC50: >2.00E+4nMAssay Description:Inhibition of recombinant C-terminal 10His-tagged human coagulation factor 10a (Leu24 to Lys488 residues) expressed in baculovirus infected Sf9 insec...More data for this Ligand-Target Pair
IC50: >2.00E+4nMAssay Description:Inhibition of recombinant C-terminal 10His-tagged human coagulation factor 10a (Leu24 to Lys488 residues) expressed in baculovirus infected Sf9 insec...More data for this Ligand-Target Pair
Prothrombin(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
IC50: >2.00E+5nMAssay Description:Inhibition of recombinant thrombin (unknown origin) using D-Phe-Pip-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition by c...More data for this Ligand-Target Pair
Prothrombin(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
IC50: >2.00E+5nMAssay Description:Inhibition of recombinant thrombin (unknown origin) using D-Phe-Pip-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition by c...More data for this Ligand-Target Pair
Prothrombin(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
IC50: >2.00E+5nMAssay Description:Inhibition of recombinant thrombin (unknown origin) using D-Phe-Pip-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition by c...More data for this Ligand-Target Pair
Prothrombin(Homo sapiens (Human))Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
IC50: >2.00E+5nMAssay Description:Inhibition of recombinant thrombin (unknown origin) using D-Phe-Pip-Arg-pNA as substrate preincubated for 30 mins followed by substrate addition by c...More data for this Ligand-Target Pair
Hemagglutinin(Influenza A virus (strain A/X-31 H3N2))SociÉTÉ
US Patent
 BDBM236493(US10988505, Comparative #2 | US9365853, 4)
BDBM236493(US10988505, Comparative #2 | US9365853, 4)Hemagglutinin(Influenza A virus (strain A/X-31 H3N2))SociÉTÉ
US Patent
 BDBM525149(US10988505, Comparative #1)
BDBM525149(US10988505, Comparative #1)EC50: 7.70E+3nMAssay Description:The ability of the tested compound to block influenza virus replication (PR8 and X31) in Calu-3 human bronchial epithelial cells was evaluated as des...More data for this Ligand-Target Pair
 BDBM525149(US10988505, Comparative #1)
BDBM525149(US10988505, Comparative #1)EC50: 5.07E+3nMAssay Description:The ability of the tested compound to block influenza virus replication (PR8 and X31) in Calu-3 human bronchial epithelial cells was evaluated as des...More data for this Ligand-Target Pair
Displayed 1 to 50 (of 106 total ) | Next | Last >>