Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB (change energy unit to kcal/mol)
 Found 49 hits in this display
Found 49 hits in this display
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
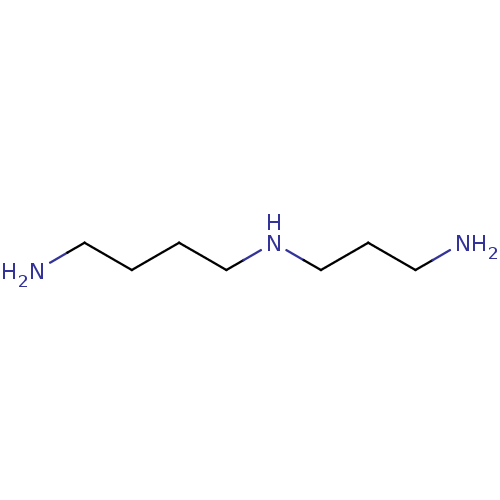 BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
 BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
 BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
 BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
 BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
 BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
 BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
 BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
 BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
 BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
 BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)Ki: 3.00E+6nMAssay Description:Competitive inhibition of bull prostate ornithine decarboxylase using DL-[1-14C]ornithine as substrate after 1 hr by dixon plot analysisMore data for this Ligand-Target Pair
 BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
BDBM50009353(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)IC50: >1.00E+6nMAssay Description:Inhibition of 3 nM recombinant human topoisomerase-2alpha catalytic activity expressed in Saccharomyces cerevisiae JEL1 harboring topoisomerase1 dele...More data for this Ligand-Target Pair
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
BDBM79403(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)
 BDBM197310(SAMPL5, CBClip)
BDBM197310(SAMPL5, CBClip)University Of California San Diego
Kd: 1.14E+6nMpH: 7.4 T: 2°CAssay Description:The experimental studies were carried out in 20 mM sodium phosphate buffer at pH7.4, at a temperature of 298 K.More data for this Ligand-Host Pair