Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB (change energy unit to kcal/mol)
 Found 88 hits in this display
Found 88 hits in this display
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
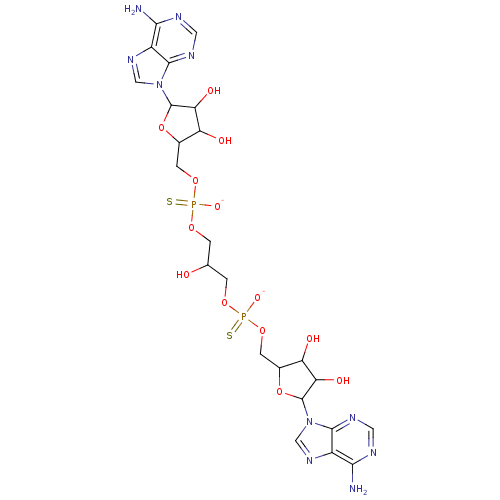 BDBM81576(AppppA analog, 6 (X=S))
BDBM81576(AppppA analog, 6 (X=S))
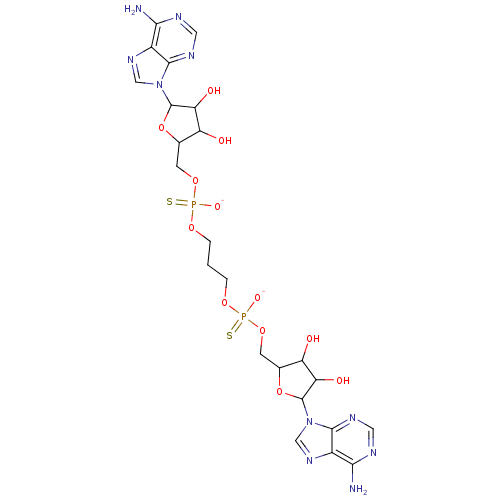 BDBM81580(AppppA analog, 9 (X=S))
BDBM81580(AppppA analog, 9 (X=S))
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
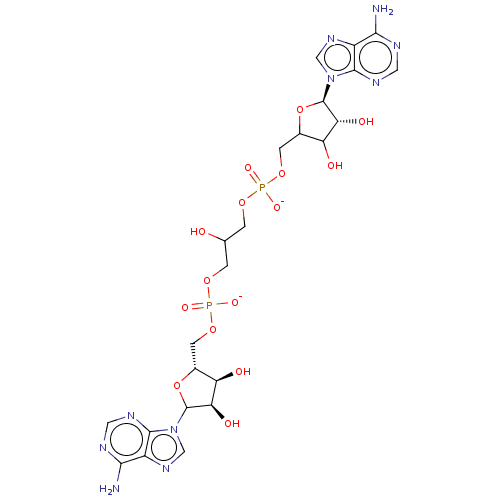 BDBM81577(AppppA analog, 6 (X=O))
BDBM81577(AppppA analog, 6 (X=O))
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
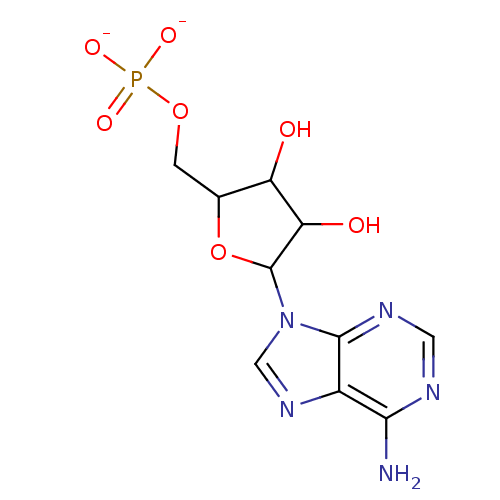 BDBM92538(AMP | [5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2...)
BDBM92538(AMP | [5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)Ki: 8.00E+4nM ΔG°: -23.4kJ/mole IC50: 8.00E+5nMpH: 7.5 T: 2°CAssay Description:Aminoacyl-tRNA synthetase assays were measuring the incorporation of [14C] amino acid into tRNA.More data for this Ligand-Target Pair
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
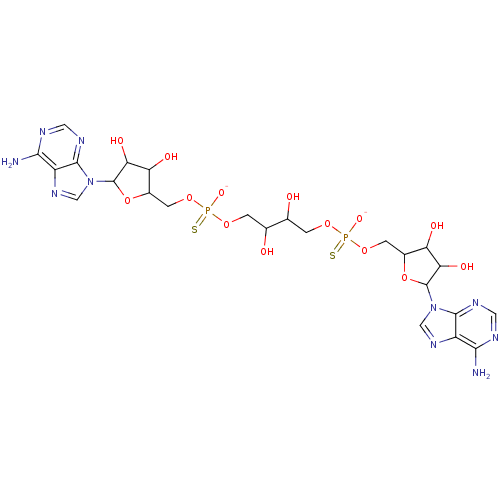 BDBM81573(AppppA analog, 4 (X=S))
BDBM81573(AppppA analog, 4 (X=S))
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
Ki: 9.80E+6nMAssay Description:Inhibitory activity against rat adenylate kinase II was determined in the presence of AMP, non competitive inhibitionMore data for this Ligand-Target Pair
Ki: 1.60E+7nMAssay Description:Inhibitory activity against rat Adenylate kinase M isoenzyme was determined in the presence of ATP, non competitive inhibitionMore data for this Ligand-Target Pair
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)IC50: 4.19E+3nMAssay Description:Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATPMore data for this Ligand-Target Pair
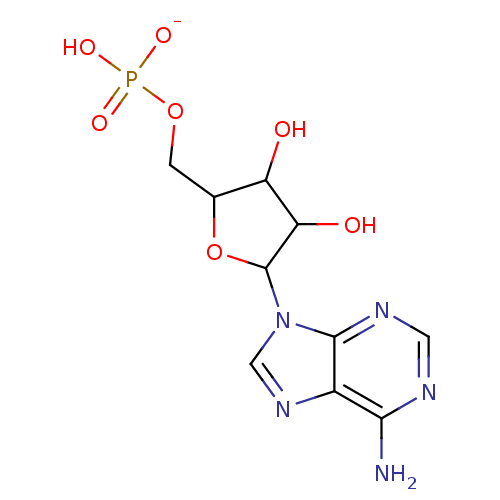 BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)
BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)
 BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)
BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)IC50: 1.32E+4nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute Ass...More data for this Ligand-Target Pair
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
 BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)
BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)IC50: 7.00E+4nMAssay Description:Inhibition of human TRPM2 assessed as reduction in ADPR-induced channel currents by whole cell patch clamp electrophysiology methodMore data for this Ligand-Target Pair
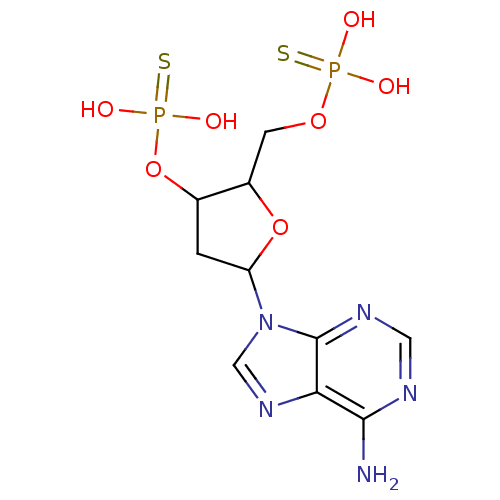 BDBM50062284(CHEMBL56787 | Thiophosphoric acid 5-(6-amino-purin...)
BDBM50062284(CHEMBL56787 | Thiophosphoric acid 5-(6-amino-purin...)IC50: 8.80E+4nMAssay Description:Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATPMore data for this Ligand-Target Pair
 BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)
BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)IC50: 1.22E+5nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute Ass...More data for this Ligand-Target Pair
 BDBM50192453(AMP(2-) | Adenosine-5-monophosphate | LDHA Inhibit...)
BDBM50192453(AMP(2-) | Adenosine-5-monophosphate | LDHA Inhibit...)
 BDBM50280244(Phosphoric acid mono-[(2R,3R,4S,5R)-5-(6-amino-pur...)
BDBM50280244(Phosphoric acid mono-[(2R,3R,4S,5R)-5-(6-amino-pur...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)EC50: 1.73E+4nMAssay Description:Measure of Agonist Potency at human P2Y purinoceptor 11 (hP2Y11) stably expressed in 131N1 astrocytoma cell at 10 uMMore data for this Ligand-Target Pair
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)
BDBM61258((5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)...)EC50: >3.00E+5nMAssay Description:Keywords: GSK3beta, dose response, kinase, inhibition, HTS Assay Overview: The glycogen synthase kinase-3 beta (GSK-3b) is a known master regulator f...More data for this Ligand-Target Pair
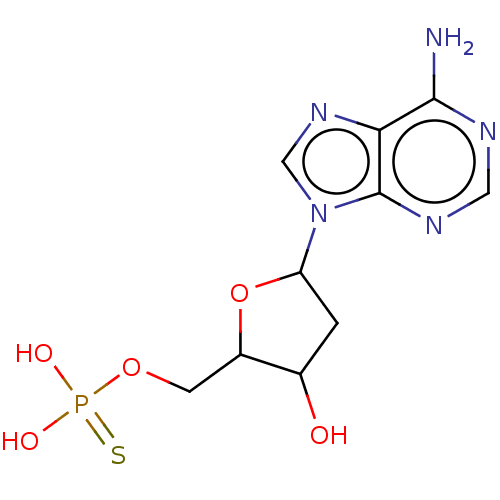 BDBM526224(US11185100, TABLE 7.10)
BDBM526224(US11185100, TABLE 7.10)
 BDBM525969(US11185100, TABLE 6.6)
BDBM525969(US11185100, TABLE 6.6)
 BDBM525969(US11185100, TABLE 6.6)
BDBM525969(US11185100, TABLE 6.6)
 BDBM525969(US11185100, TABLE 6.6)
BDBM525969(US11185100, TABLE 6.6)
 BDBM525969(US11185100, TABLE 6.6)
BDBM525969(US11185100, TABLE 6.6)
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)EC50: >1.00E+4nMAssay Description:HEK293 cells that stably express T1R3 and inducibly express T1R1 were exposed to nucleotide derivatives alone to activate the umami receptor. Activat...More data for this Ligand-Target Pair
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)EC50: >3.00E+4nMAssay Description:HEK293 cells that stably express T1R3 and inducibly express T1R1 were exposed to nucleotide derivatives alone to activate the umami receptor. Activat...More data for this Ligand-Target Pair
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)EC50: >3.00E+4nMAssay Description:HEK293 cells that stably express T1R3 and inducibly express T1R1 were exposed to nucleotide derivatives alone to activate the umami receptor. Activat...More data for this Ligand-Target Pair
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
 BDBM526098(US11185100, TABLE 7.2)
BDBM526098(US11185100, TABLE 7.2)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM526218(US11185100, TABLE 7.9)
BDBM526218(US11185100, TABLE 7.9)
 BDBM50192453(AMP(2-) | Adenosine-5-monophosphate | LDHA Inhibit...)
BDBM50192453(AMP(2-) | Adenosine-5-monophosphate | LDHA Inhibit...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)EC50: 2.34E+3nMAssay Description:Concentration at which 50% of the maximal effect (stimulation of PLC at P2Y1 receptor in the turkey erythrocyte membranes) is reachedMore data for this Ligand-Target Pair
 BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)
BDBM50062279(CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...)EC50: 1.28E+3nMAssay Description:Agonist activity at P2Y1 receptor measured as capacity to stimulate 50% phospholipase C in turkey erythrocyte membranesMore data for this Ligand-Target Pair
 BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
BDBM18137(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)
Displayed 1 to 50 (of 88 total ) | Next | Last >>