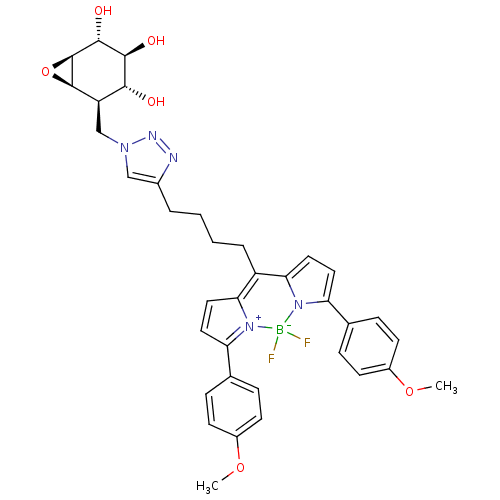
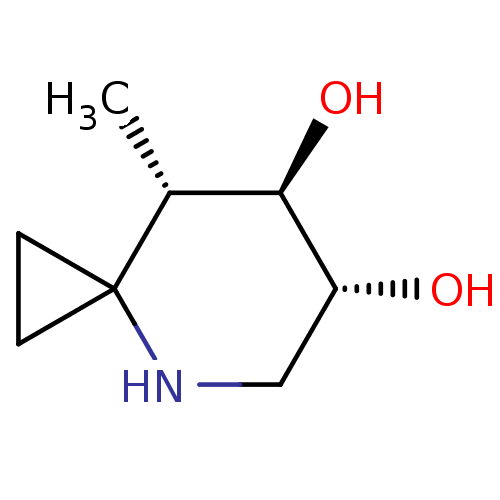
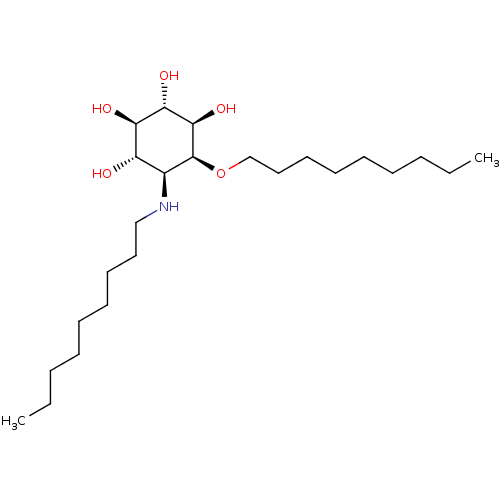
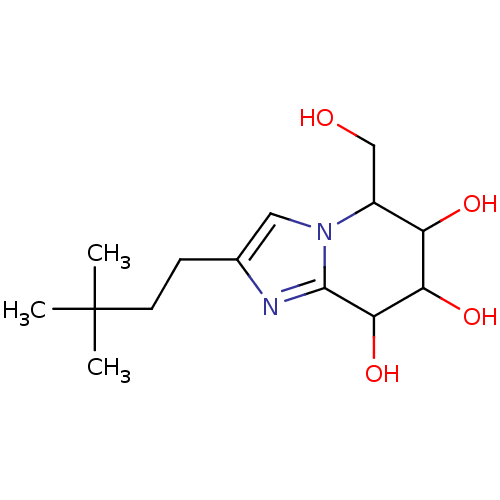
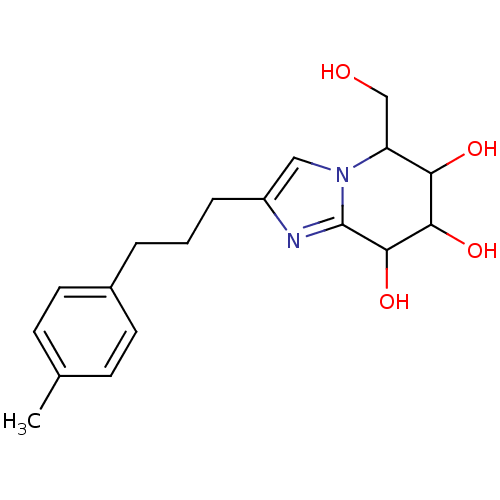
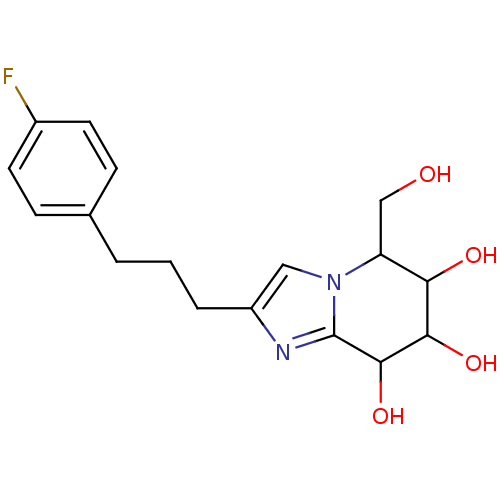
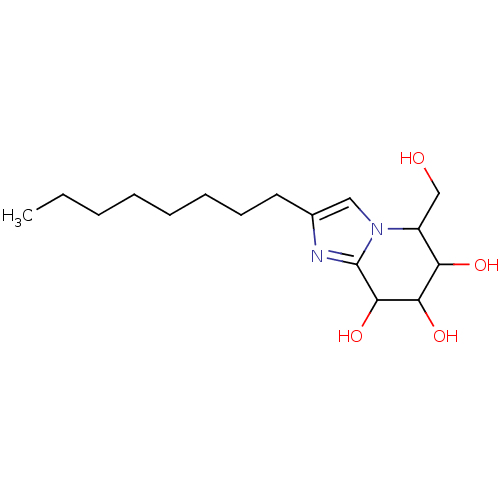
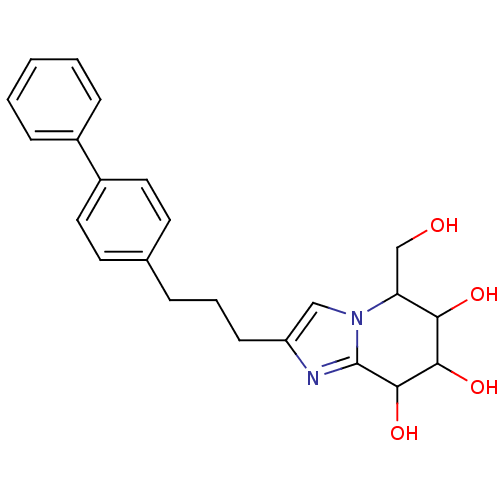
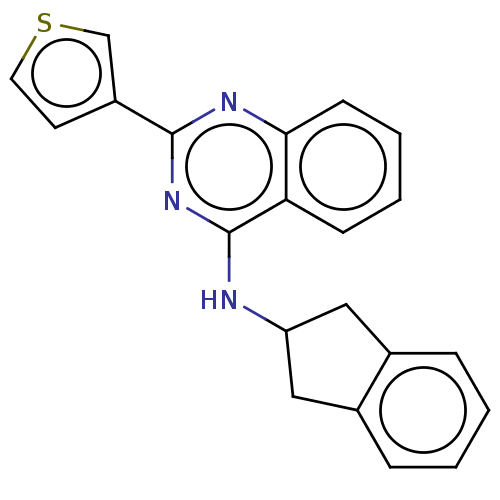
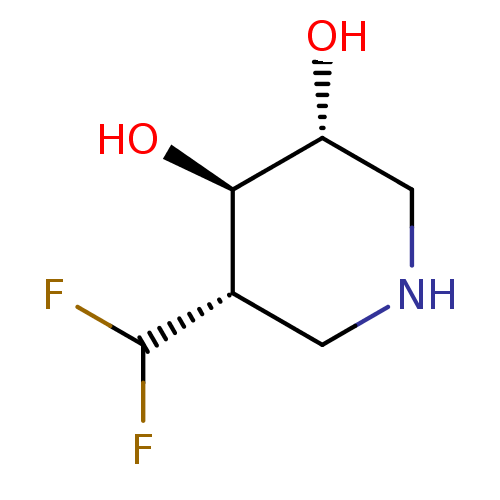
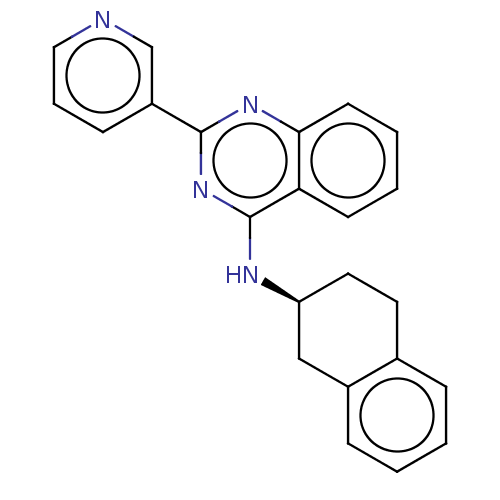
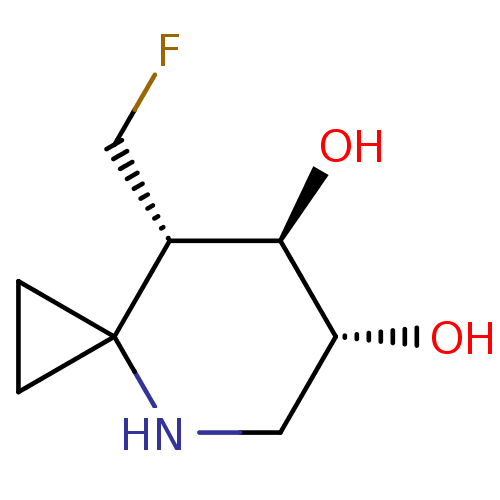
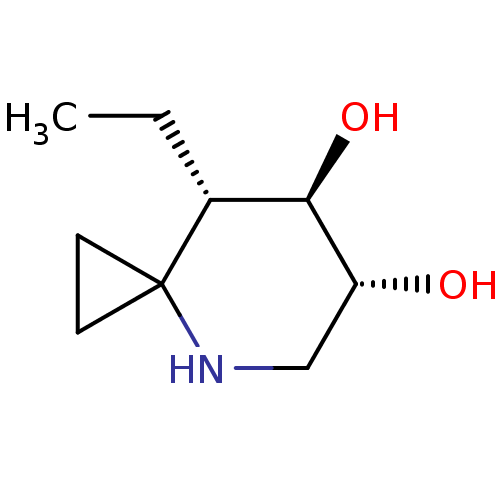
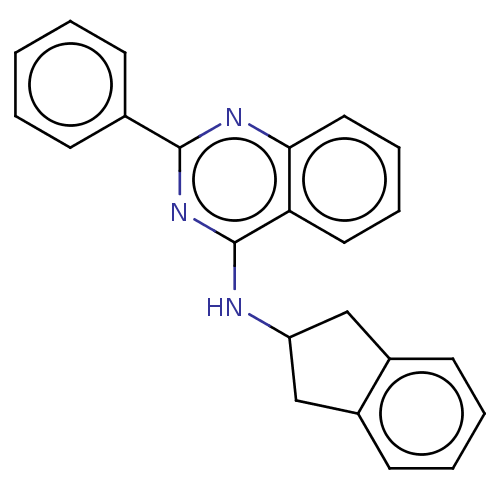
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of human GCase assessed as reduction of 4-methylumbelliferone liberation using 4-methylumbelliferyl-beta-glucopyranoside as substrate incu...More data for this Ligand-Target Pair
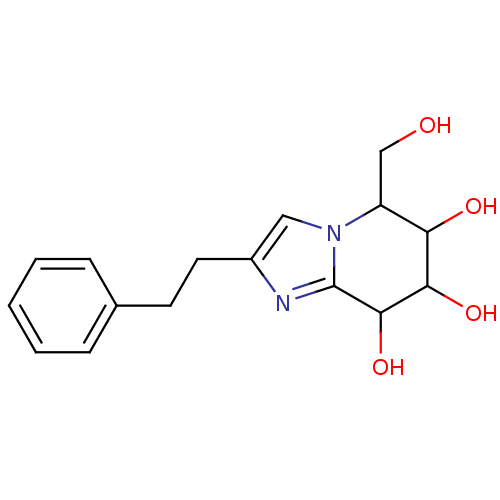
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 7nM ΔG°: -11.6kcal/mole IC50: 1.24nMpH: 5.2 T: 2°CAssay Description:Enzymatic activity assay using fluorescent activity-based labeling method.More data for this Ligand-Target Pair
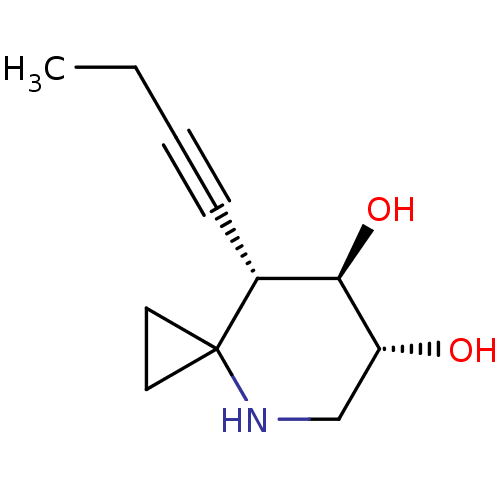
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 8nM ΔG°: -11.5kcal/mole IC50: 1.94nMpH: 5.2 T: 2°CAssay Description:Enzymatic activity assay using fluorescent activity-based labeling method.More data for this Ligand-Target Pair
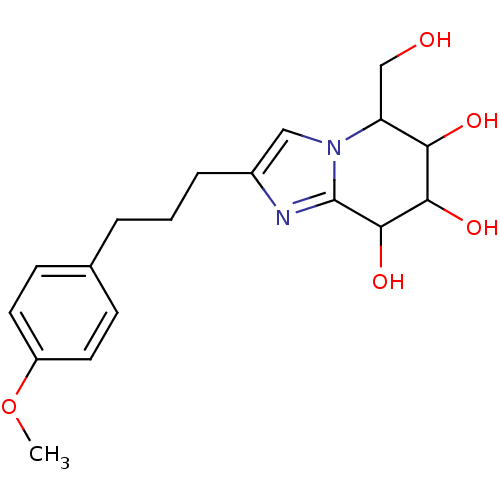
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.95nMAssay Description:The enzyme in GCase enzyme activity buffer (25 μL/well) was added to a 384-well black plate. The fluorescent probe 3 (25 nL/well, 50 nM final co...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 0.950nM IC50: 2nMpH: 5.2Assay Description:Activity of GBA was measured at 37° C. with 4-methylumbelliferyl β-D-glucopyranoside as substrate as reported previously. To determine the IC50 ...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.04nMpH: 7.0Assay Description:The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.40nMpH: 7.0Assay Description:Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.5nMpH: 7.0Assay Description:Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.5nMAssay Description:Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.97nMpH: 5.2Assay Description:The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 3.62nMpH: 7.0Assay Description:The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 3.70nMAssay Description:Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Ligand Info
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 3.70nMpH: 7.0Assay Description:Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 3.71nMpH: 5.2Assay Description:The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 4nMpH: 5.2Assay Description:Competitive inhibition of recombinant Imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before sub...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 4nMpH: 5.2Assay Description:Competitive inhibition of recombinant Imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before sub...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 4.26nMpH: 7.0Assay Description:The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 4.30nMAssay Description:Inhibition of lysosomal beta-glucosidase in human fibroblast using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 24 hrs b...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 4.73nMpH: 7.0Assay Description:The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 4.75nMpH: 7.0Assay Description:The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 5nMpH: 7.0Assay Description:Competitive inhibition of recombinant Imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before sub...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 5nMpH: 7.0Assay Description:Competitive inhibition of recombinant Imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before sub...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 5.05nMpH: 7.0Assay Description:The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 5.11nMpH: 7.0Assay Description:The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 5.20nMAssay Description:The enzyme in GCase enzyme activity buffer (25 μL/well) was added to a 384-well black plate. The fluorescent probe 3 (25 nL/well, 50 nM final co...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 5.20nMAssay Description:The compounds in DMSO solution (0.5 μL/well) were transferred to a black 96-well plate (the final titration started from 100 μM, a 12 or 24...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 5.20nMpH: 5.9Assay Description:Inhibition of synthetic recombinant wild type GCase enzyme velaglucerase alfa (unknown origin) at pH 5.9 preincubated for 5 mins followed by addition...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 5.27nMpH: 5.2Assay Description:The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 5.34nMpH: 5.2Assay Description:The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 5.44nMpH: 5.2Assay Description:The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 5.60nMAssay Description:The enzyme in GCase enzyme activity buffer (25 μL/well) was added to a 384-well black plate. The fluorescent probe 3 (25 nL/well, 50 nM final co...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 3.06nM IC50: 5.80nMpH: 7.0Assay Description:The enzyme inhibition assays used monitored the ability of a test compound to bind and prevent the hydrolysis of a fluorogenic substrate in a concent...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:The enzyme in GCase enzyme activity buffer (25 μL/well) was added to a 384-well black plate. The fluorescent probe 3 (25 nL/well, 50 nM final co...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:The enzyme in GCase enzyme activity buffer (25 μL/well) was added to a 384-well black plate. The fluorescent probe 3 (25 nL/well, 50 nM final co...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 6.20nMAssay Description:Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 6.20nMpH: 7.0Assay Description:Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 6.23nMpH: 5.2Assay Description:The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 6.30nMAssay Description:Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 6.30nMpH: 7.0Assay Description:Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 6.5nMAssay Description:The compounds in DMSO solution (0.5 μL/well) were transferred to a black 96-well plate (the final titration started from 100 μM, a 12 or 24...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
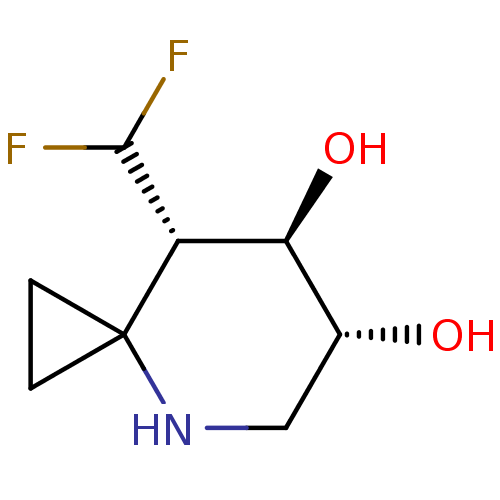
Ligand Info
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 6.5nMAssay Description:The enzyme in GCase enzyme activity buffer (25 μL/well) was added to a 384-well black plate. The fluorescent probe 3 (25 nL/well, 50 nM final co...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 6.5nMAssay Description:Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 6.5nMpH: 5.9Assay Description:Inhibition of synthetic recombinant wild type GCase enzyme velaglucerase alfa (unknown origin) at pH 5.9 preincubated for 5 mins followed by addition...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 6.5nMpH: 7.0Assay Description:Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau...More data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 6.60nMAssay Description:The compounds in DMSO solution (0.5 μL/well) were transferred to a black 96-well plate (the final titration started from 100 μM, a 12 or 24...More data for this Ligand-Target Pair