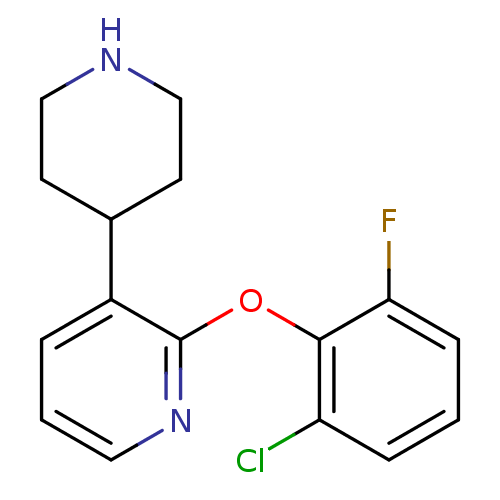
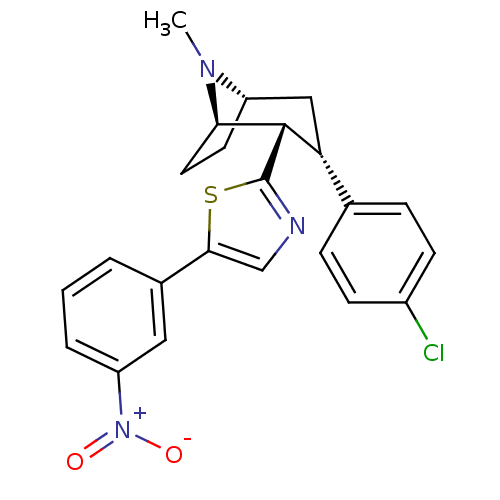
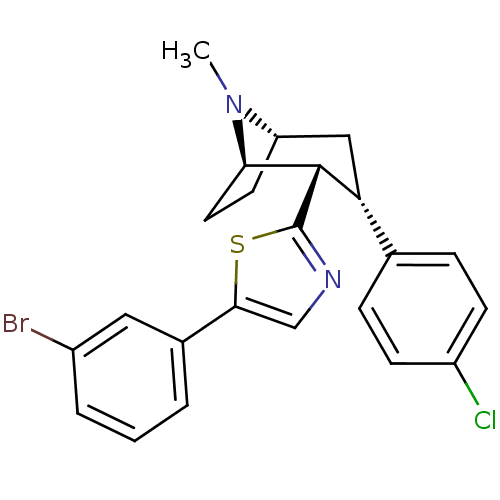
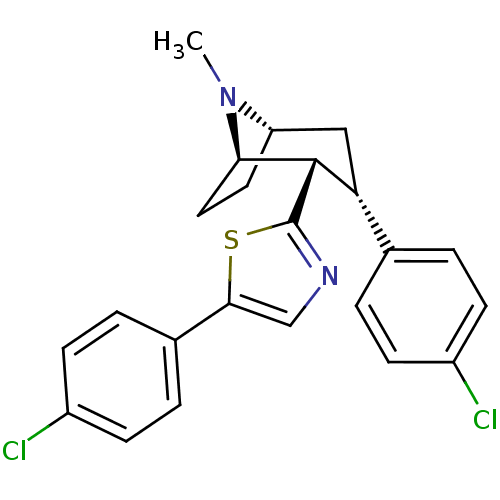
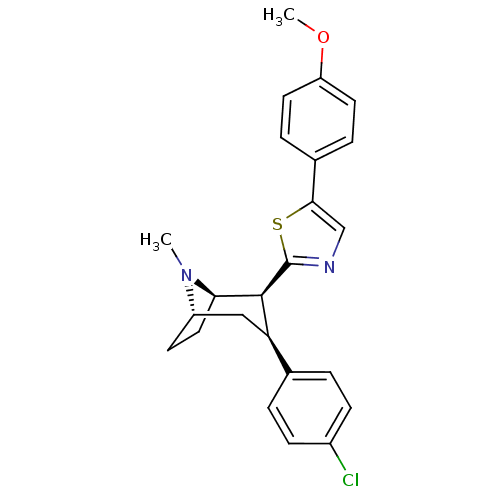
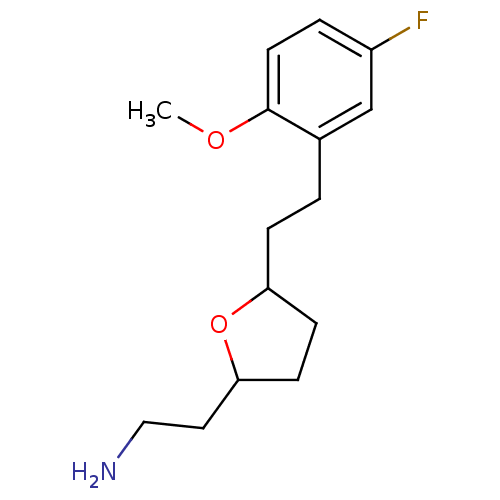
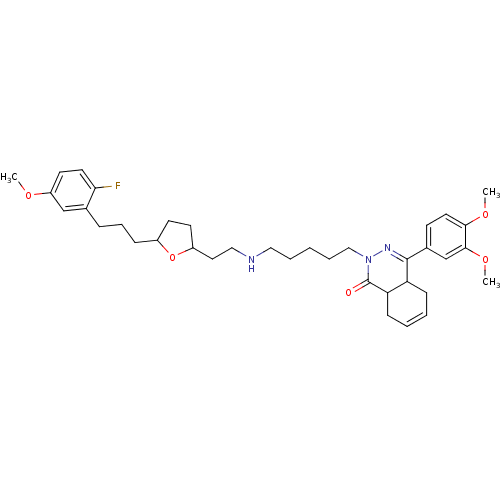
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 1.10nM ΔG°: -12.1kcal/mole EC50: 2.30nMpH: 7.4 T: 2°CAssay Description:Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ...More data for this Ligand-Target Pair
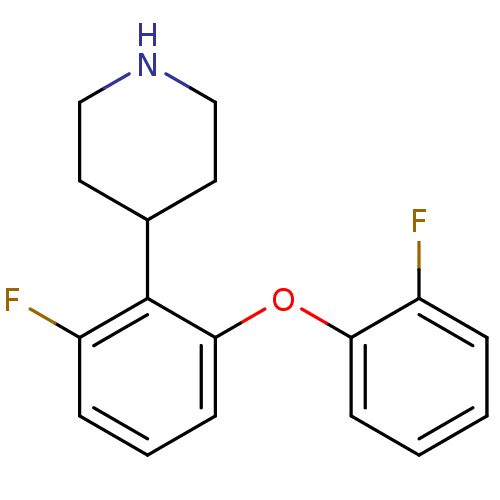
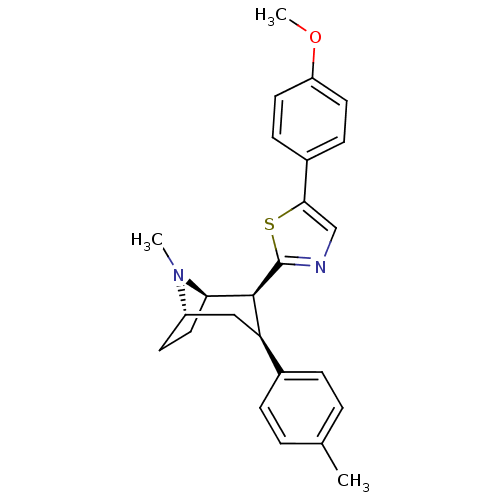
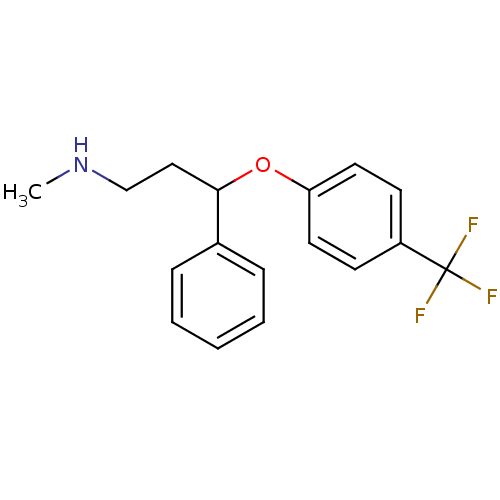
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 2.10nM ΔG°: -11.7kcal/mole EC50: 2.5nMpH: 7.4 T: 2°CAssay Description:Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ...More data for this Ligand-Target Pair
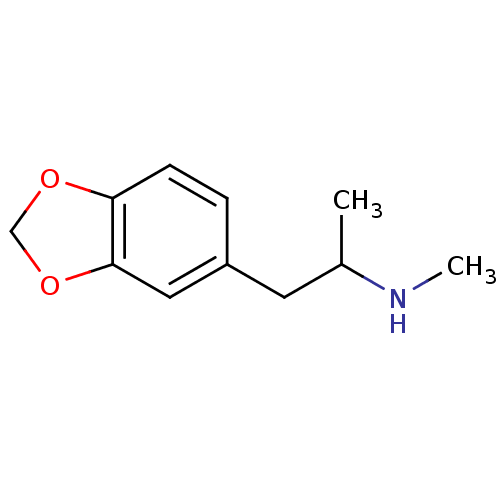
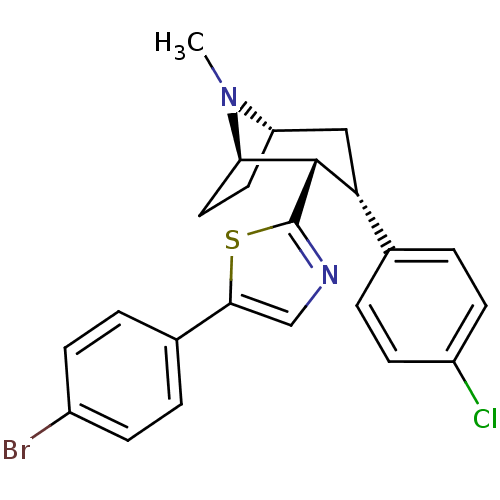
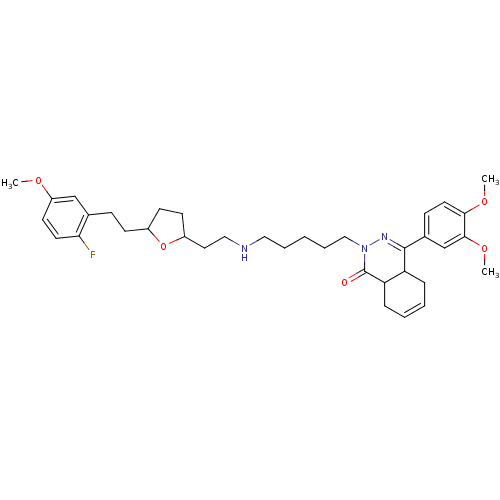
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 2.70nMAssay Description:Inhibition of human SERT-mediated serotonin reuptake in HEK293 cellsMore data for this Ligand-Target Pair
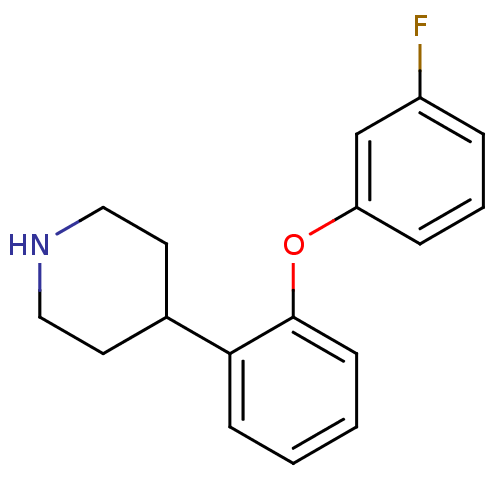
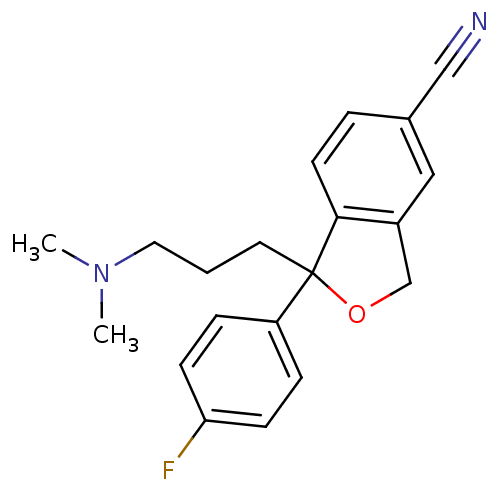
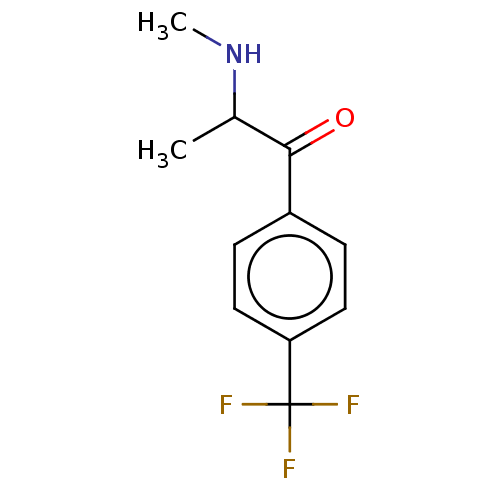
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 1.10nM ΔG°: -12.1kcal/mole EC50: 7.30nMpH: 7.4 T: 2°CAssay Description:Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 21.2nM ΔG°: -10.4kcal/mole EC50: 18.4nMpH: 7.4 T: 2°CAssay Description:Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 118nMAssay Description:Inhibition of [3H]dopamine uptake in HEK cells expressing human Dopamine transporterMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 156nM ΔG°: -9.19kcal/mole EC50: 127nMpH: 7.4 T: 2°CAssay Description:Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 186nMAssay Description:Inhibition of [3H]5-HT uptake at human 5HTT expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 190nMAssay Description:Activity at SERT (unknown origin) assessed as release of [3H]HTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 194nM ΔG°: -9.06kcal/mole EC50: 194nMpH: 7.4 T: 2°CAssay Description:Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 601nM EC50: 318nMAssay Description:Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 1.32E+3nM EC50: 377nMAssay Description:Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 409nMAssay Description:Agonist activity at SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 276nM ΔG°: -8.85kcal/mole EC50: 416nMpH: 7.4 T: 2°CAssay Description:Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 915nMAssay Description:Agonist activity at SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 915nMAssay Description:Inhibition of [3H]5-HT uptake at human 5HTT expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 1.00E+3nMAssay Description:Binding affinity to human SERT expressed in HEK293 cells assessed as induction of [3H]5-HT release by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 653nM EC50: 1.01E+3nMAssay Description:Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 1.02E+3nMAssay Description:Inhibition of [3H]5-HT uptake at human 5HTT expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 1.60E+3nMAssay Description:Inhibition of [3H]-S-citalopram dissociation from human SERT allosteric modulator site (S2) expressed in African green monkey COS7 cell membranes aft...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 740nM EC50: 1.61E+3nMAssay Description:Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 1.76E+3nMAssay Description:Inhibition of [3H]5-HT uptake at human 5HTT expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 1.77E+3nMAssay Description:Inhibition of [3H]5-HT uptake at human SERT cloned in rat brain synaptosome by scintillation countingMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 1.87E+3nM EC50: 1.84E+3nMAssay Description:Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 2.12E+3nMAssay Description:Induction of SERT (unknown origin)-mediated serotonin releaseMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 580nM EC50: 2.38E+3nMAssay Description:Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 2.67E+3nM EC50: 2.71E+3nMAssay Description:Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 2.90E+3nMAssay Description:Inhibition of [3H]-S-citalopram dissociation from human SERT allosteric modulator site (S2) expressed in African green monkey COS7 cell membranes aft...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 2.93E+3nMAssay Description:Inhibition of [3H]5-HT uptake at human 5HTT expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 3.16E+3nMAssay Description:Decrease in [3H]5-HT uptake at human SERT G498C mutant transfected in HEK293 cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 1.64E+3nM EC50: 3.17E+3nMAssay Description:Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 1.72E+3nM EC50: 3.20E+3nMAssay Description:Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 1.82E+3nM EC50: 3.60E+3nMAssay Description:Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 870nM EC50: 3.86E+3nMAssay Description:Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 3.86E+3nMAssay Description:Activity at SERT (unknown origin) assessed as release of [3H]HTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 3.98E+3nMAssay Description:Decrease in [3H]5-HT uptake at human SERT G498C mutant transfected in HEK293 cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 1.50E+3nM EC50: 4.00E+3nMAssay Description:Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 2.39E+3nM EC50: 4.40E+3nMAssay Description:Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 2.94E+3nM EC50: 4.40E+3nMAssay Description:Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 5.10E+3nMAssay Description:Inhibition of [3H]-S-citalopram dissociation from SERT allosteric modulator site (S2) (unknown origin) expressed in African green monkey COS1 cell me...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 2.71E+3nM EC50: 5.30E+3nMAssay Description:Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 5.80E+3nMAssay Description:Inhibition of [3H]-S-citalopram dissociation from human SERT allosteric modulator site (S2) expressed in African green monkey COS7 cell membranes aft...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 5.90E+3nMAssay Description:Inhibition of [3H]-S-citalopram dissociation from human SERT allosteric modulator site (S2) expressed in African green monkey COS7 cell membranes aft...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 5.20E+3nM EC50: 6.70E+3nMAssay Description:Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 3.59E+3nM EC50: >6.80E+3nMAssay Description:Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 3.67E+3nM EC50: 6.80E+3nMAssay Description:Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 7.70E+3nMAssay Description:Inhibition of [3H]-S-citalopram dissociation from human SERT allosteric modulator site (S2) expressed in African green monkey COS7 cell membranes aft...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataKi: 5.70E+3nM EC50: 8.22E+3nMAssay Description:Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 8.70E+3nMAssay Description:Inhibition of [3H]-S-citalopram dissociation from SERT allosteric modulator site (S2) (unknown origin) expressed in African green monkey COS1 cell me...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Human Biomolecular Research Institute
Human Biomolecular Research Institute
Affinity DataEC50: 8.80E+3nMAssay Description:Inhibition of [3H]-S-citalopram dissociation from SERT allosteric modulator site (S2) (unknown origin) expressed in African green monkey COS1 cell me...More data for this Ligand-Target Pair





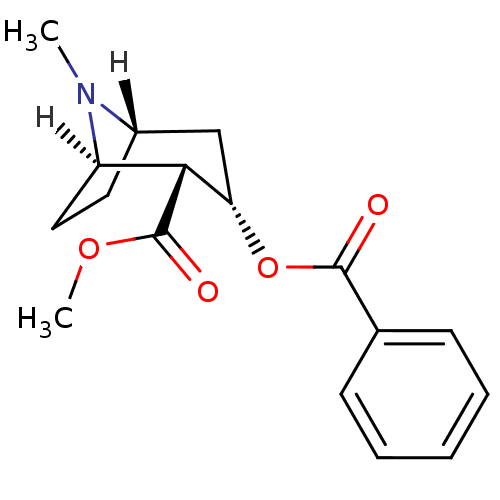
 3D Structure (crystal)
3D Structure (crystal)