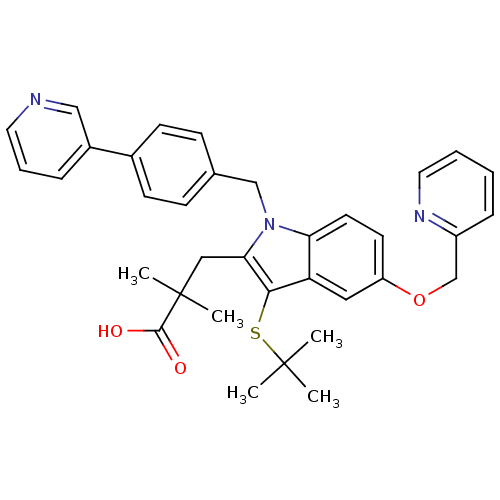
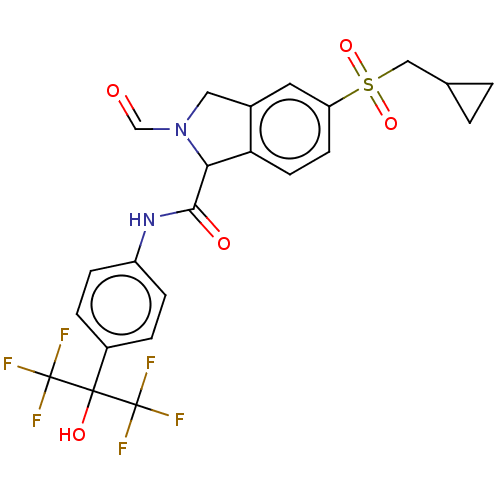
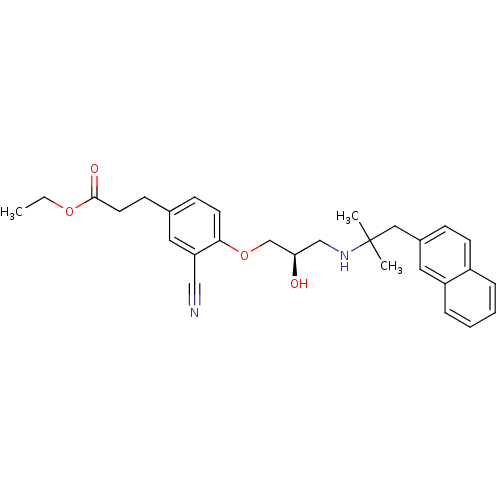
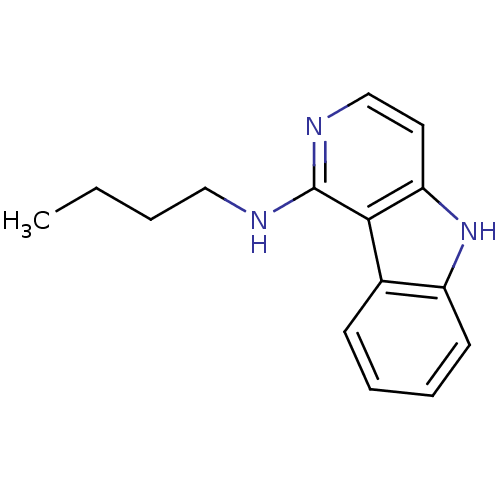
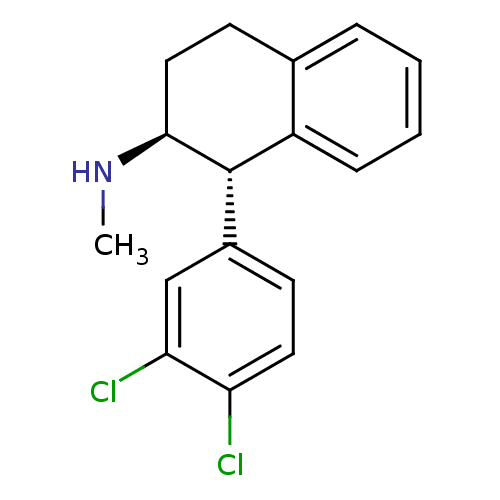
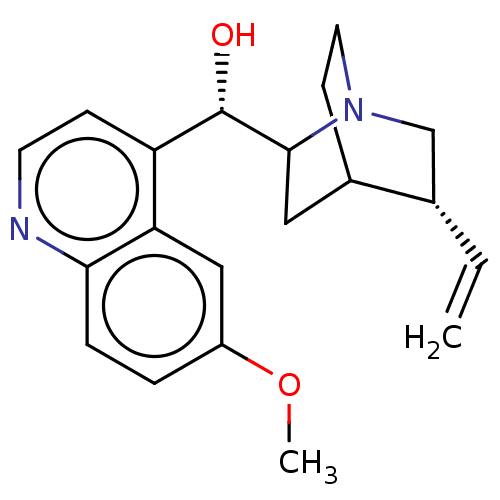
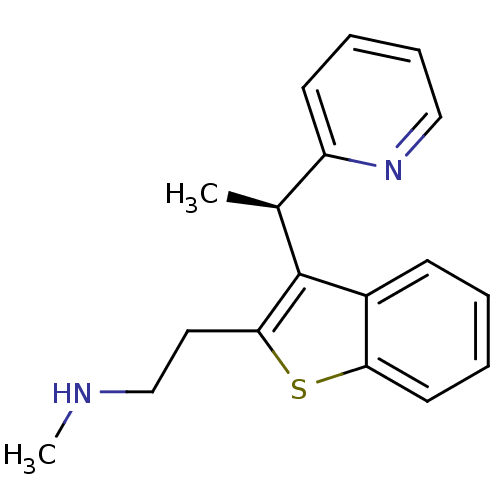
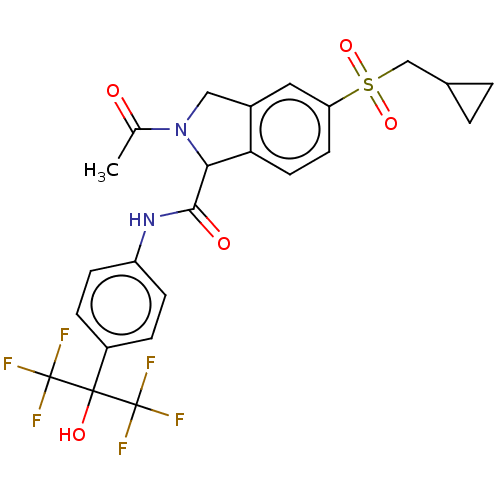
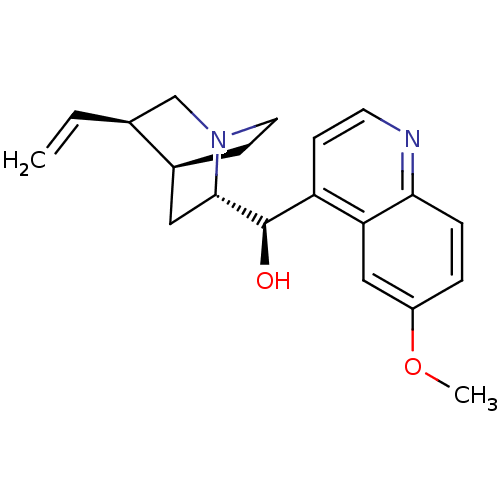
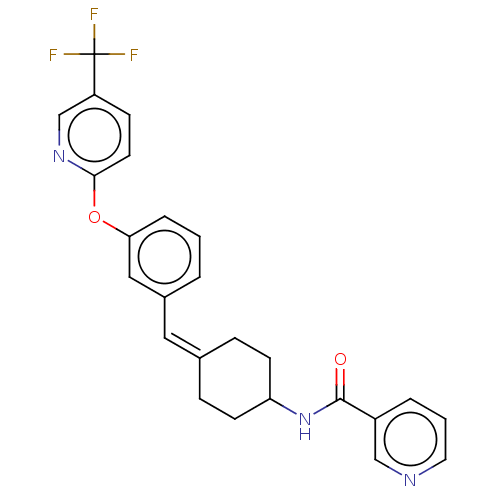
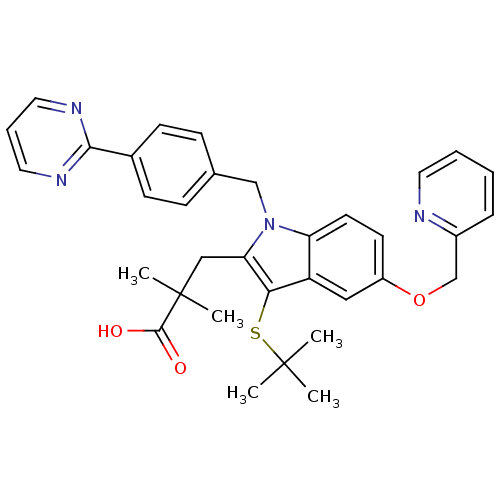
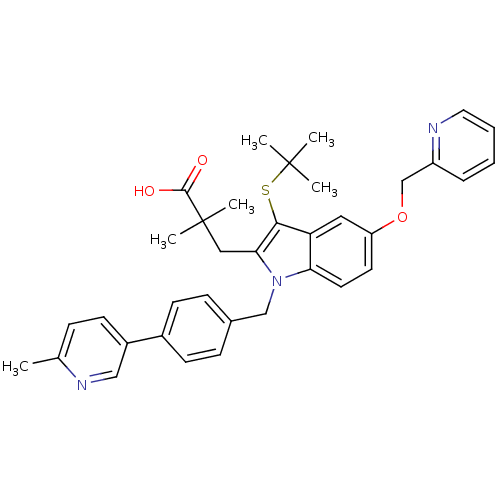
Affinity DataIC50: 0.0450nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 0.590nMAssay Description:Inhibition of CYP2D6 in human liver microsomes after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of CYP2D6 (unknown origin) using EOMCC as a substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human recombinant microsomal CYP2D6 using {3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methylcoumarin} as substrate assessed as ...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant CYP2D6 after 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of CYP2D6 in human liver microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 3.30nMAssay Description:Inhibition of MAMC O-dealkylation mediated by human Cytochrome P450 2D6 expressed in human lymphoblastoid cell lineMore data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: <6nMAssay Description:Inhibition of CYP2D6 in human liver microsomeMore data for this Ligand-Target Pair
Affinity DataIC50: 6.90nMAssay Description:Inhibition of human recombinant CYP2D6 using luciferin as substrate preincubated for 10 mins followed by substrate addition and measured after 50 min...More data for this Ligand-Target Pair
Affinity DataIC50: 7.30nMAssay Description:Inhibition of CYP2D6 (unknown origin) expressed in insect cell microsomes using dibenzylfluorescein substrate by fluorescence based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.94nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 8.10nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 8.40nMAssay Description:Inhibitory concentration against cytochrome P450 2D6More data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 10 mins by LC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMT: 2°CAssay Description:The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w...More data for this Ligand-Target Pair
Affinity DataIC50: 9.67nMAssay Description:The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso...More data for this Ligand-Target Pair
Affinity DataIC50: 9.80nMAssay Description:Inhibition of recombinant CYP2D6 after 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of CYP2D6 in human liver microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human CYP2D6 preincubated for 5 mins followed by NADPH addition and measured after 45 mins by luminescence based microplate reader anal...More data for this Ligand-Target Pair
Affinity DataIC50: >10nMAssay Description:Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate preincubated for 5 mins followed by NADPH cofactor addition and me...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human CYP2D6 by fluorescence methodMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human CYP2D6 by fluorescence methodMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human CYP2D6 in presence of NADPH by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human CYP2D6 in presence of NADPH by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of CYP2D6 after 30 mins by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibitory concentration against cytochrome P450 2D6More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human recombinant CYP2D6 incubated for 5 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of CYP2D6 (unknown origin) after 2 hrs by firefly luciferase-luminescence based P450-glo assayMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibitory activity against CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of CYP2D6 in human liver microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: >13nMAssay Description:Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate preincubated for 5 mins followed by NADPH cofactor addition and me...More data for this Ligand-Target Pair
Affinity DataIC50: >13nMAssay Description:Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate preincubated for 5 mins followed by NADPH cofactor addition and me...More data for this Ligand-Target Pair
Affinity DataIC50: >13nMAssay Description:Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate preincubated for 5 mins followed by NADPH cofactor addition and me...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of CYP2D6 using dextromethorphan as substrateMore data for this Ligand-Target Pair

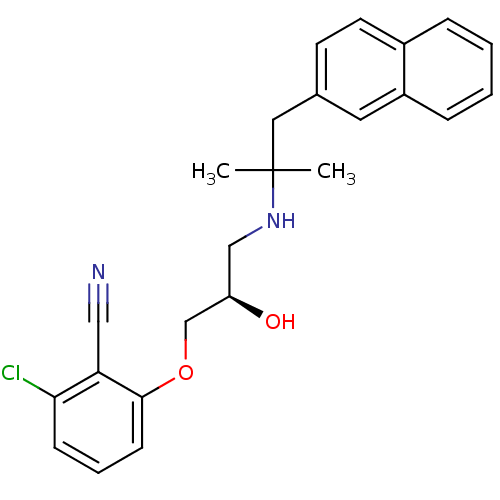
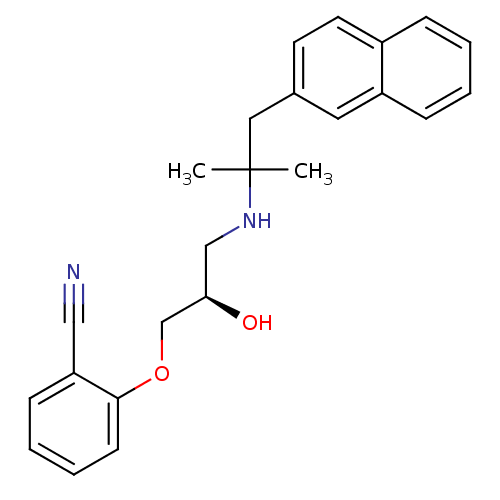
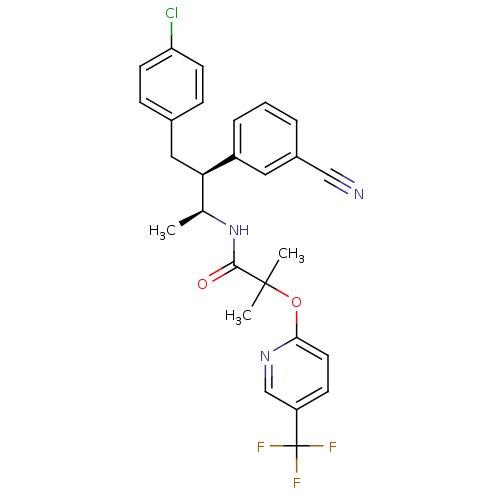
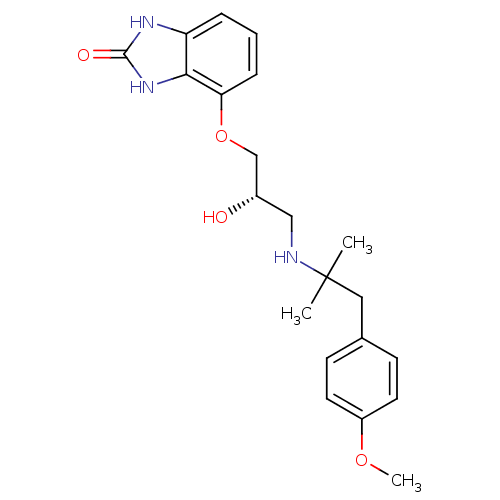
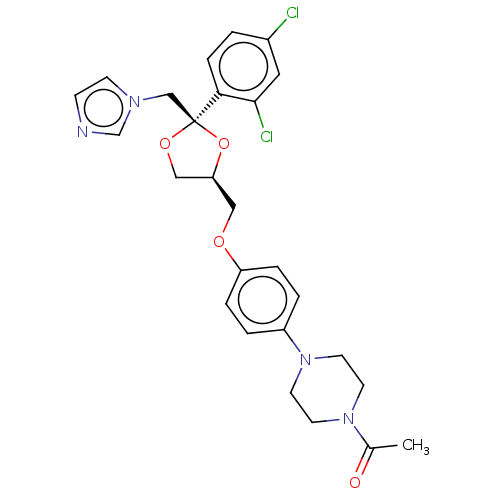
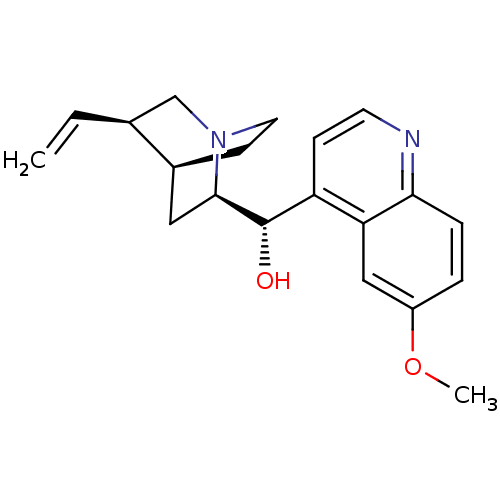
 3D Structure (crystal)
3D Structure (crystal)