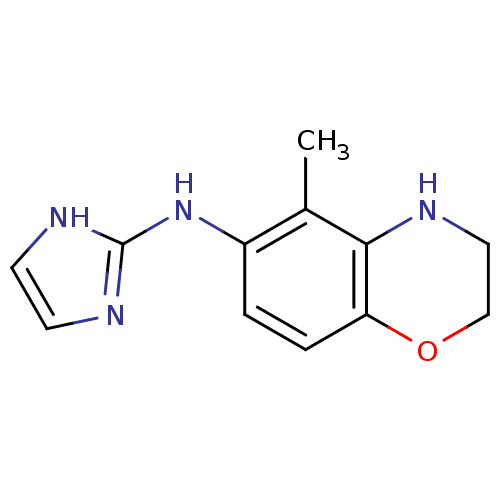
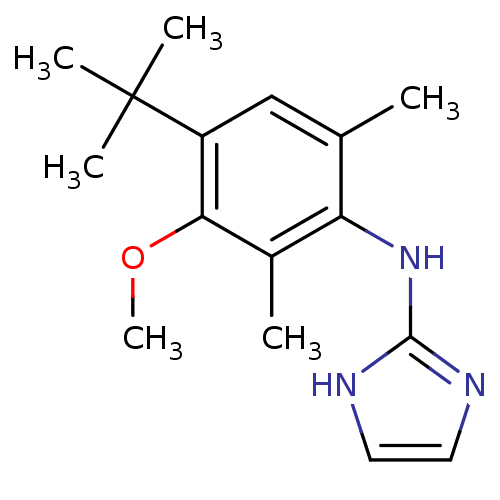
Affinity DataEC50: 41nMAssay Description:Effective Concentration at Alpha-2B adrenergic receptor from CHO-RNG cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 55nMAssay Description:Effective Concentration at Alpha-2B adrenergic receptor from CHO-RNG cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 470nMAssay Description:Effective Concentration at Alpha-2B adrenergic receptor from CHO-RNG cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 1.10E+3nMAssay Description:Effective Concentration at Alpha-2B adrenergic receptor from CHO-RNG cellsMore data for this Ligand-Target Pair