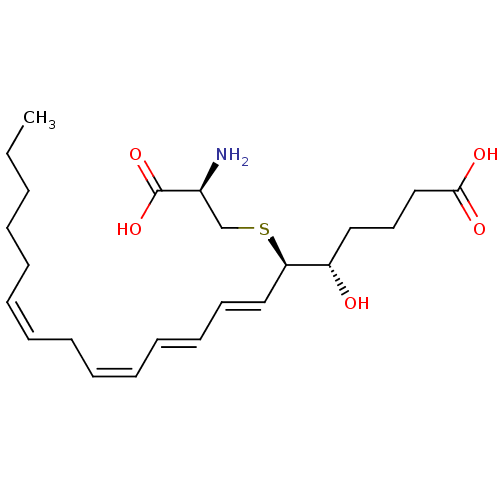
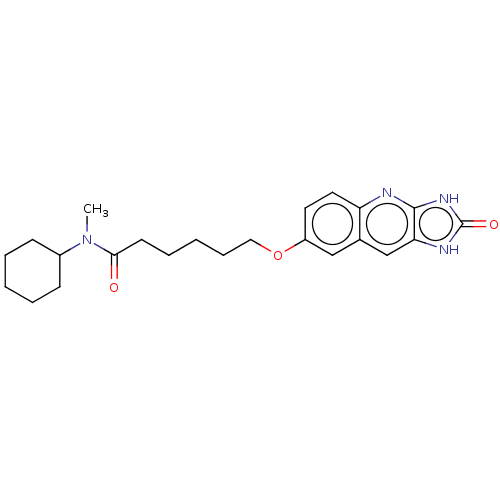
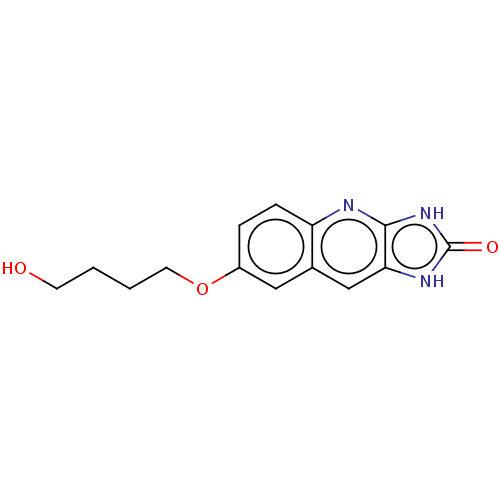
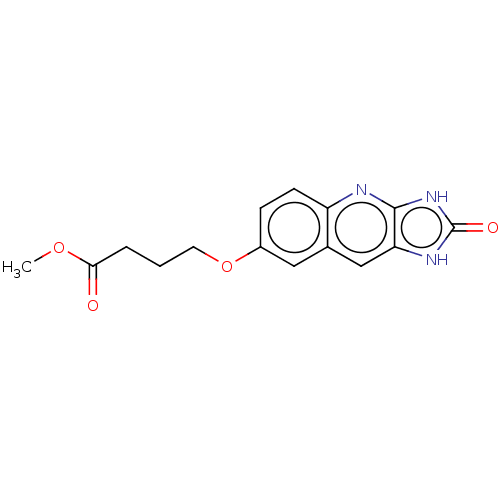
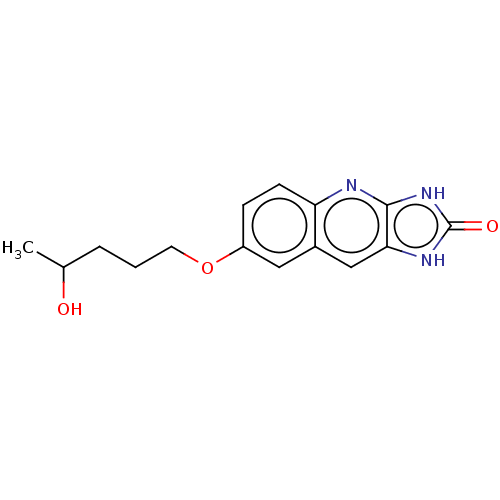
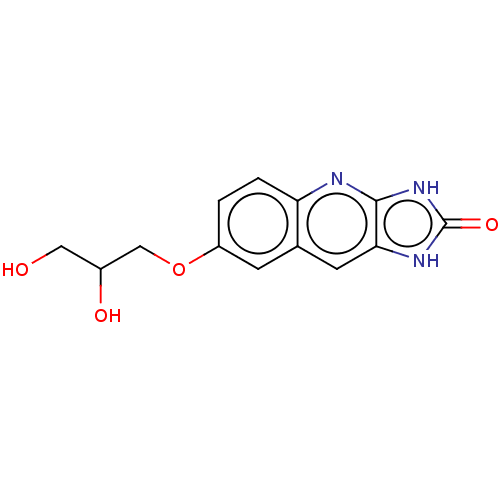
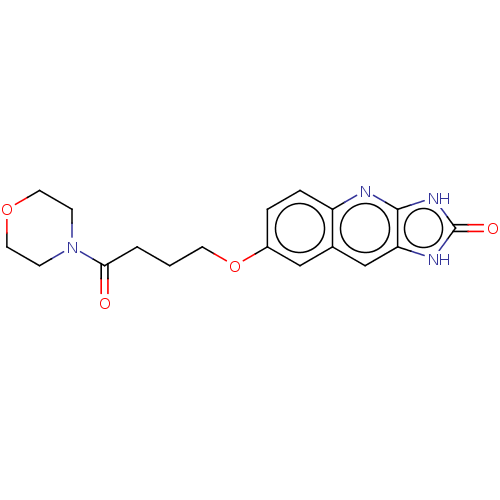
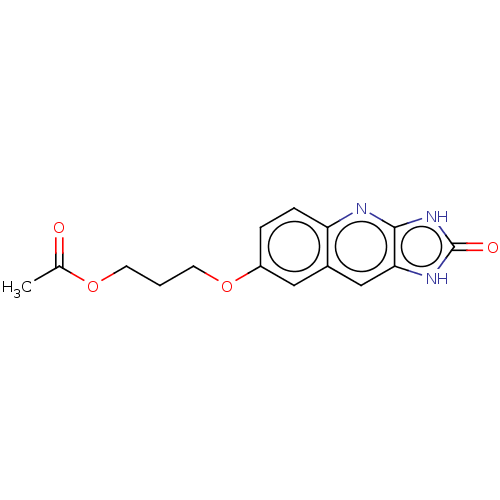
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 0.700nMAssay Description:Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 1nMAssay Description:Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 1.30nMAssay Description:Agonist activity at human P2Y12 receptorMore data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 1.30nMAssay Description:The compound was evaluated for antagonist activity against platelet P2Y purinoceptor 12 (P2Y12)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 5nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 6nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 7nMAssay Description:Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 7nMAssay Description:Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 8nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 8nMAssay Description:Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 10nMAssay Description:Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 16nMAssay Description:Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 20nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 20nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 20nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 20nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 20nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 20nMAssay Description:Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 38nMAssay Description:Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 40nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 50nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 55nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 55nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 60nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 60nMAssay Description:Activation of human P2Y12RMore data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 66nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 70nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 83nMAssay Description:Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma PRPMore data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 90nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 90nMAssay Description:Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 100nMAssay Description:Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 100nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 120nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 130nMAssay Description:Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 130nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 140nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 150nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 180nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 180nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 180nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 190nMAssay Description:Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 210nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 210nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 250nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 260nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 260nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 280nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 330nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 360nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 380nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair