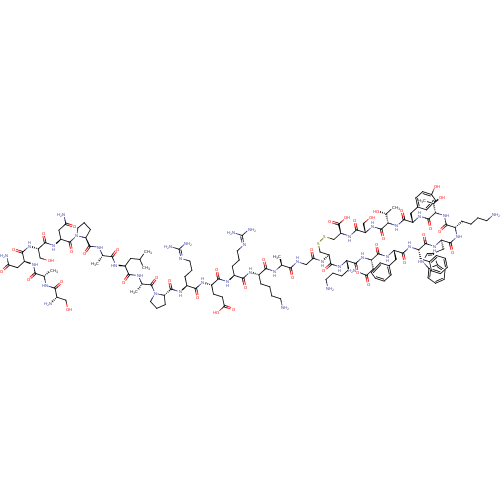
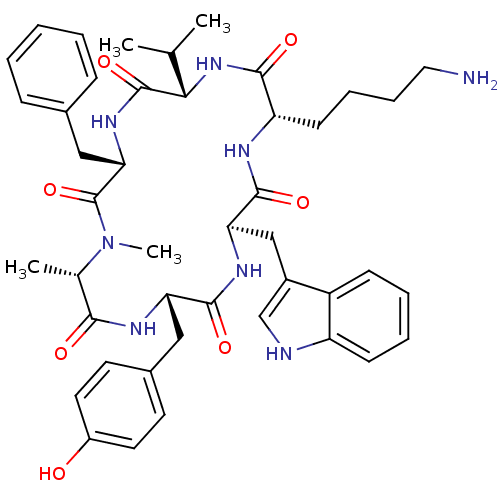
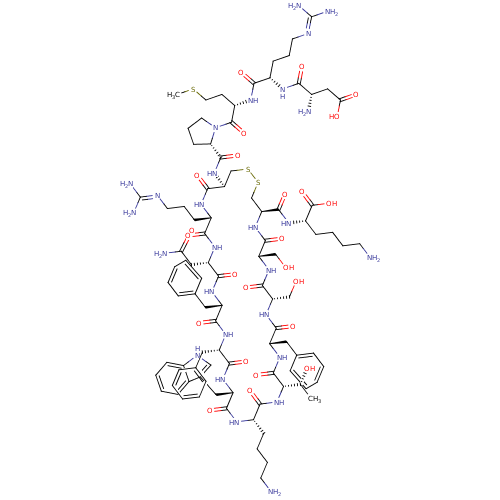
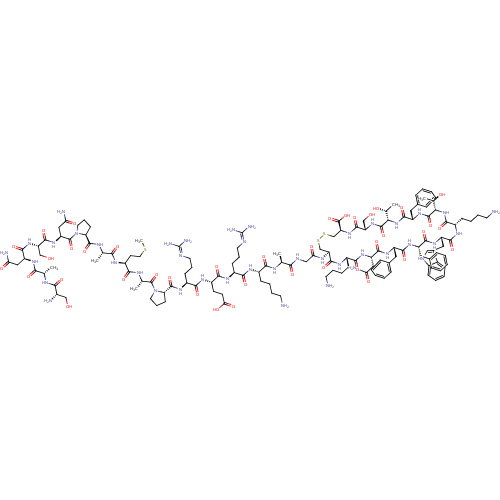
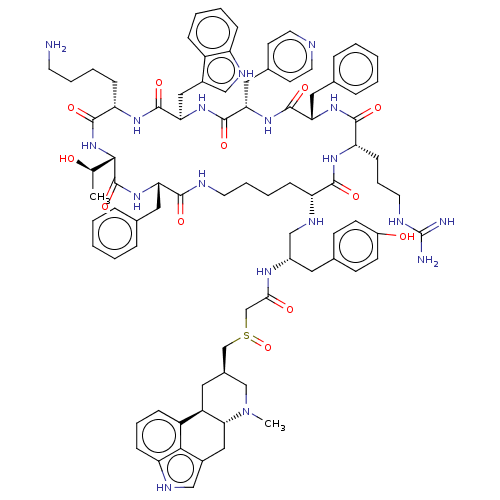
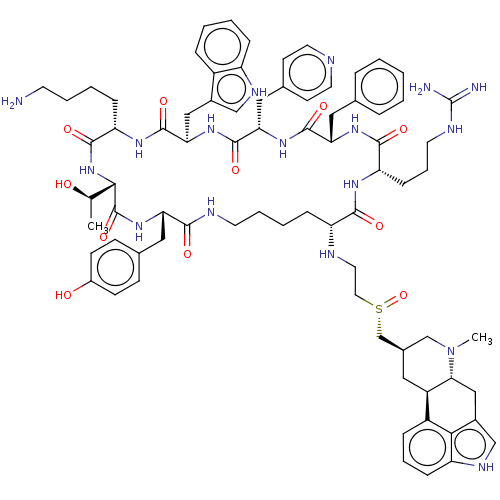
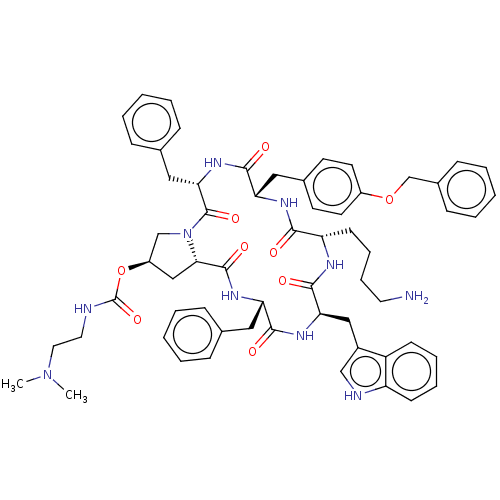
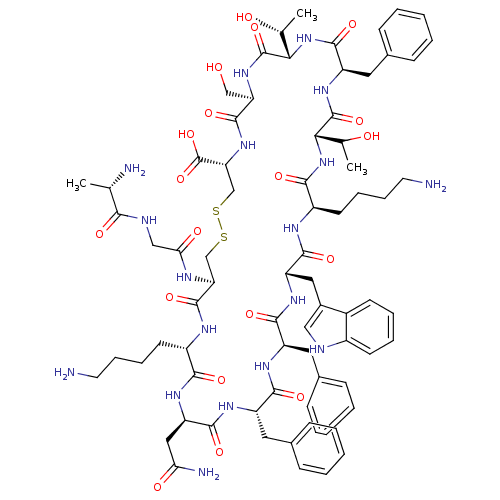
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
Affinity DataKi: 0.0800nM ΔG°: -13.8kcal/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
Affinity DataKi: 0.0900nM ΔG°: -13.7kcal/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
Affinity DataKi: 0.100nMAssay Description:Binding affinity towards human Somatostatin receptor type 5 (sst5) using Tyr11-[125I]-SRIF as radioligand was determined in COS cellMore data for this Ligand-Target Pair
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
Affinity DataKi: 0.126nMAssay Description:Binding affinity towards human Somatostatin receptor type 5 (sst5) using Tyr11-[125I]-SRIF as radioligand was determined in COS cellMore data for this Ligand-Target Pair
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
Affinity DataKi: 0.140nMAssay Description:Inhibition of human sst5 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
Affinity DataKi: 0.158nMAssay Description:Binding affinity towards human Somatostatin receptor type 5 (sst5) using Tyr11-[125I]-SRIF as radioligand was determined in COS cellMore data for this Ligand-Target Pair
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
Affinity DataKi: 0.160nM ΔG°: -13.4kcal/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
Affinity DataKi: 0.200nMAssay Description:Displacement of [125I]-somatostatin from human SSTR5 expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
Affinity DataKi: 0.230nM ΔG°: -13.1kcal/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
Affinity DataKi: 0.230nMAssay Description:Displacement of [125I]-somatostatin from human SSTR5 expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
Affinity DataKi: 0.251nMAssay Description:Binding affinity towards human Somatostatin receptor type 5 (sst5) using Tyr11-[125I]-SRIF as radioligand was determined in COS cellMore data for this Ligand-Target Pair
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
Affinity DataKi: 0.260nMAssay Description:Binding affinity against human sst5 receptor at a dose of 10 uMMore data for this Ligand-Target Pair
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Mcgill University
Curated by PDSP Ki Database
Mcgill University
Curated by PDSP Ki Database