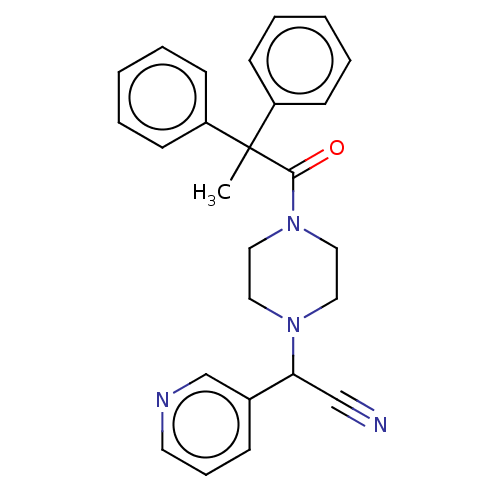
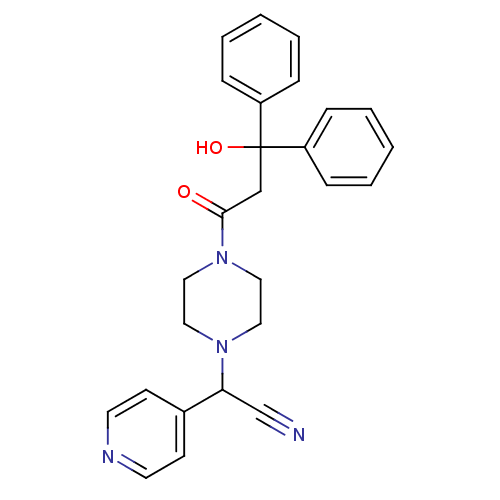
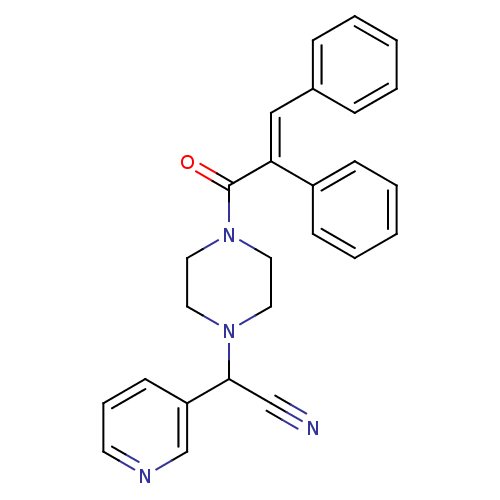
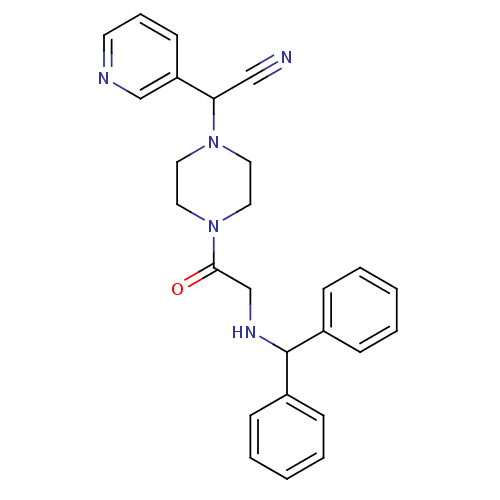
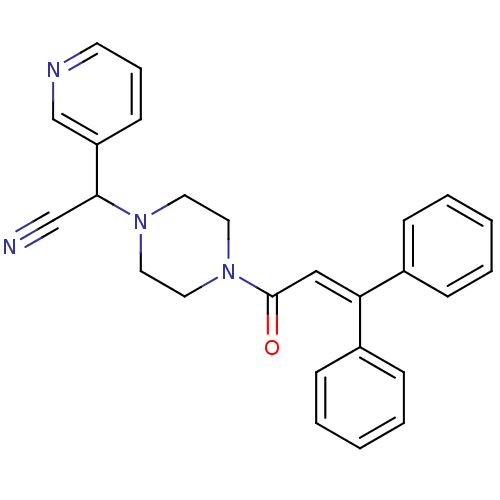
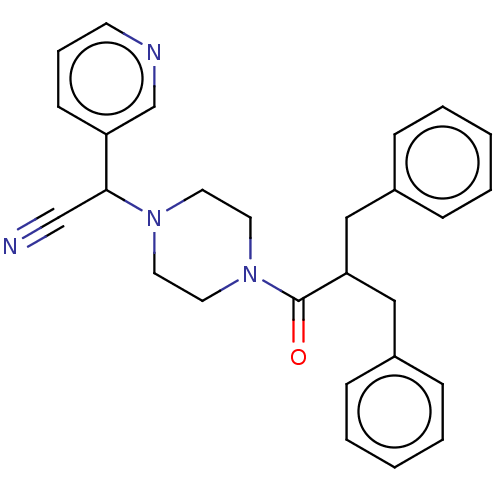
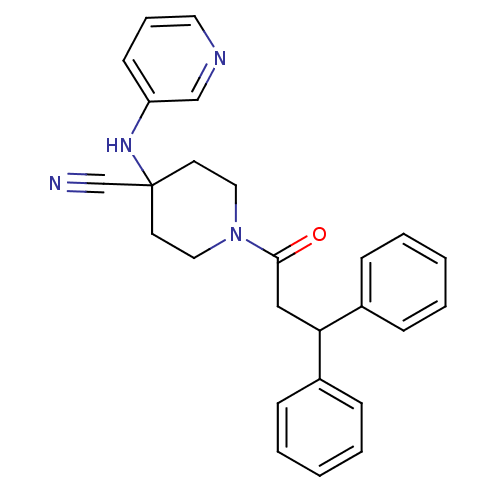
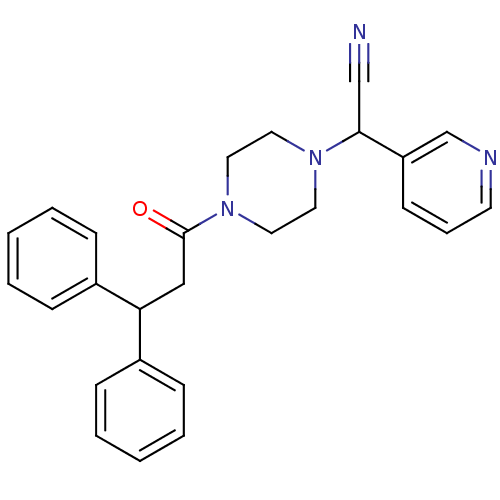
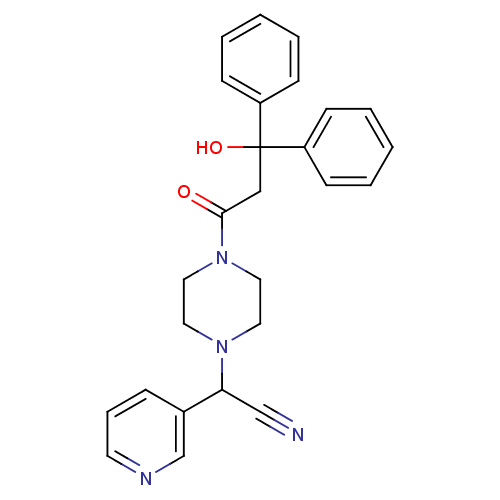
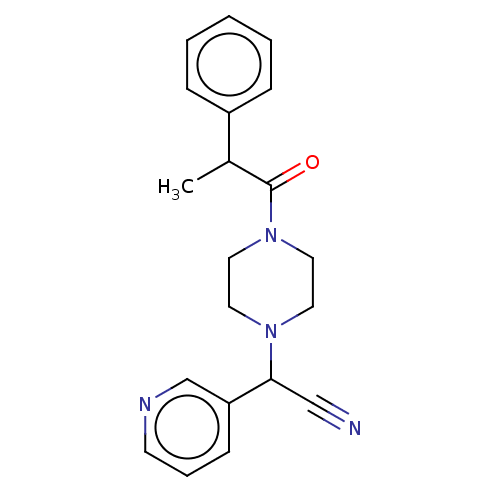
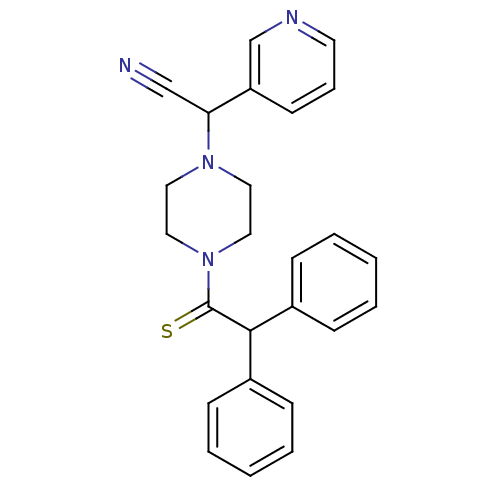
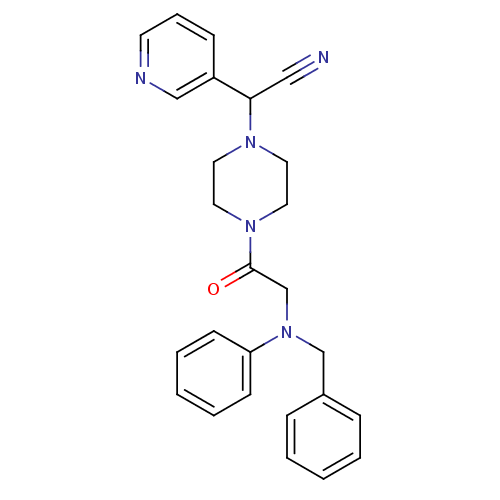
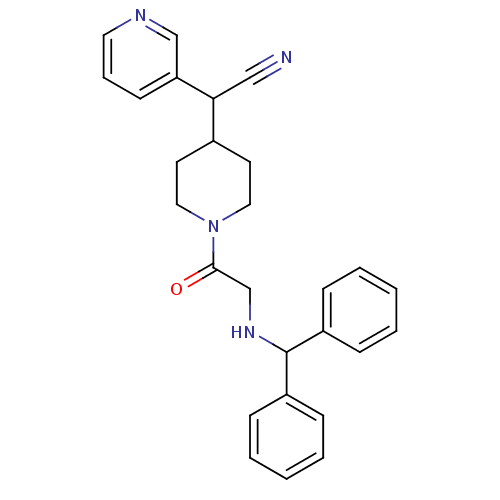
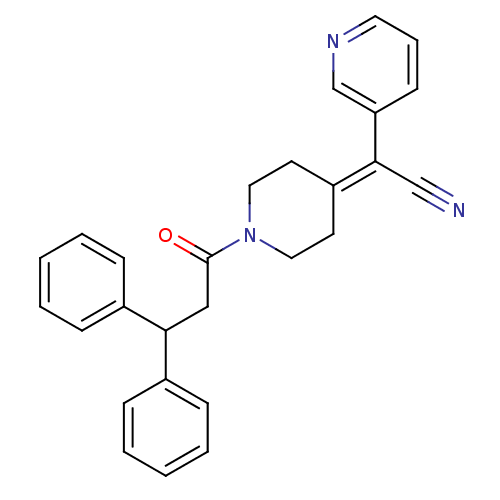
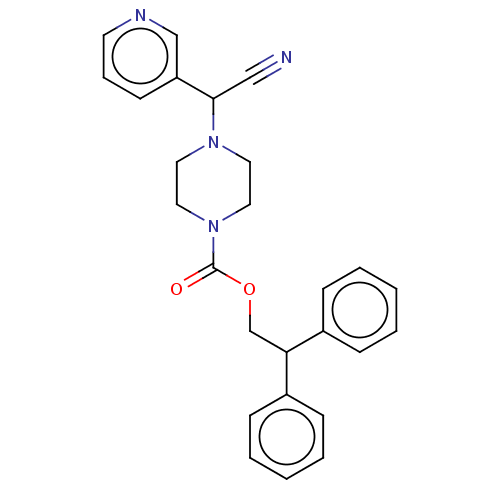
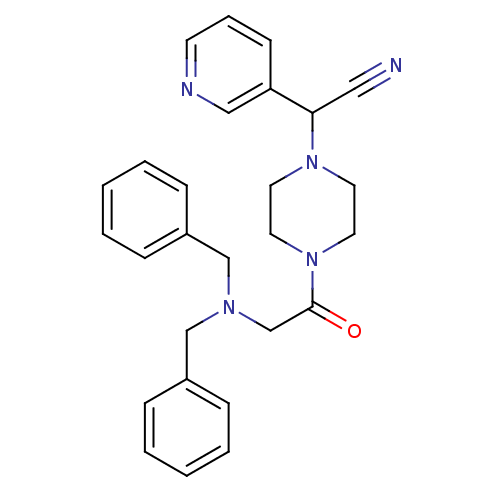
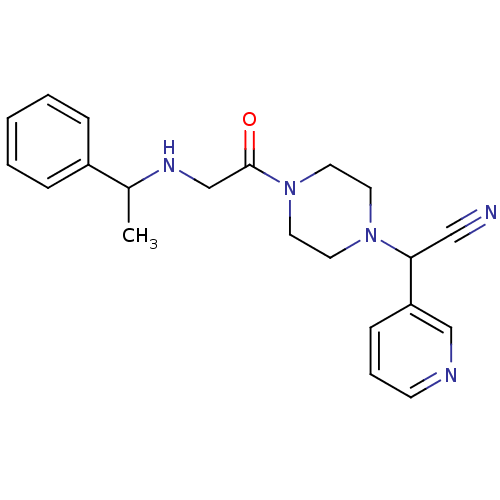
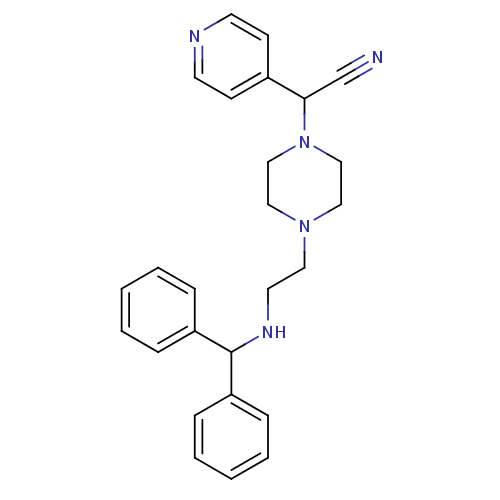
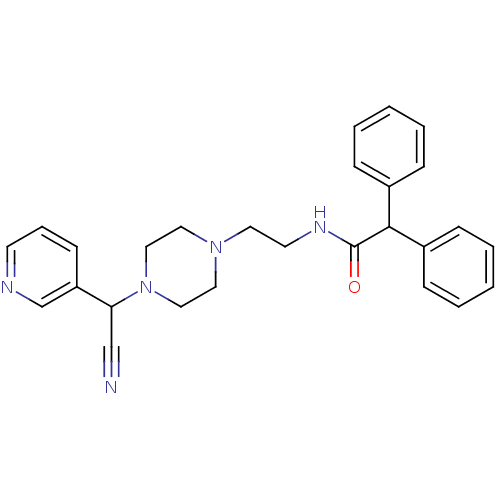
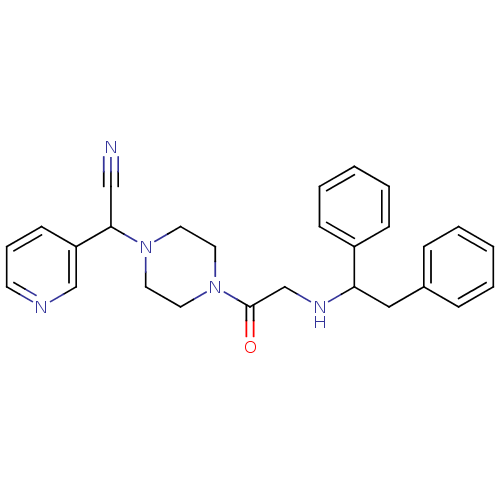
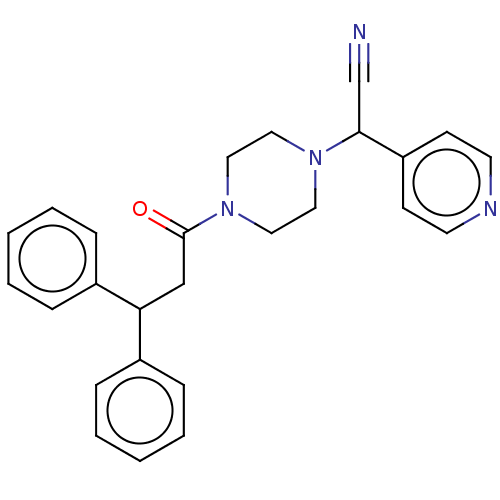
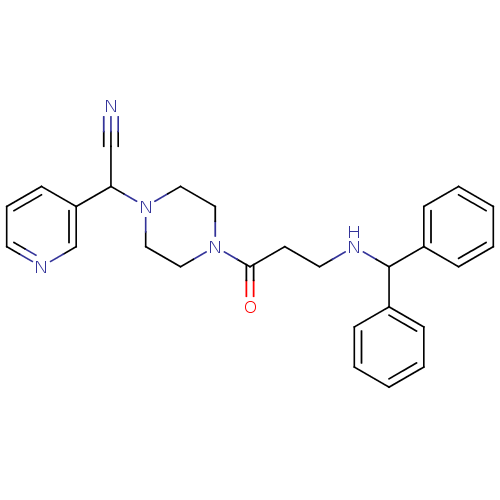
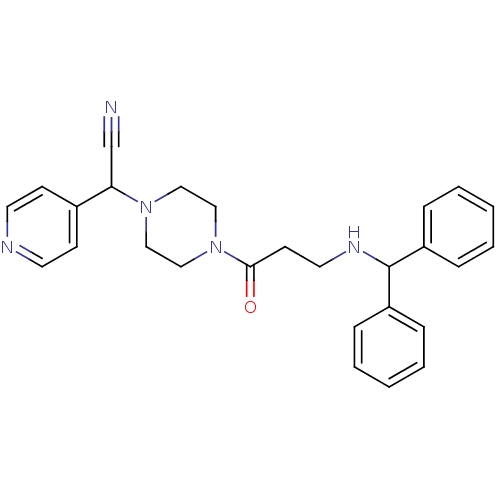
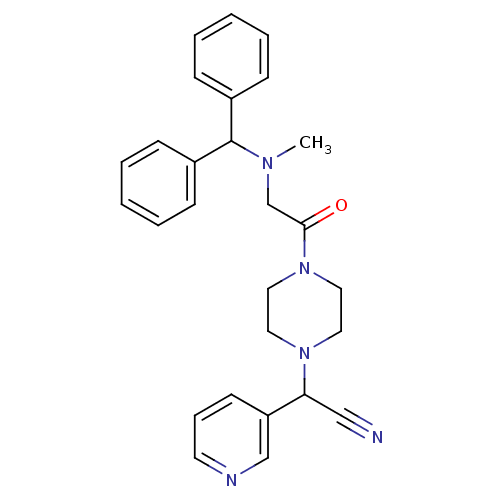
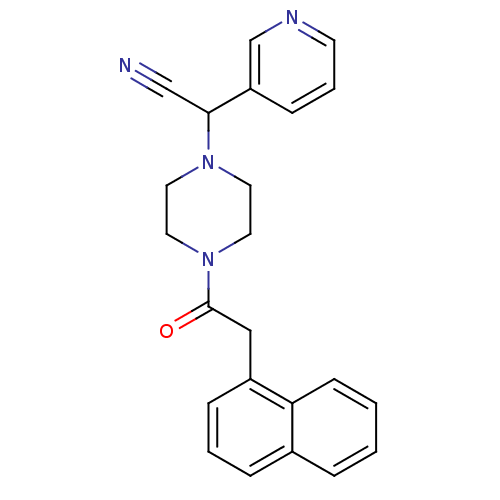
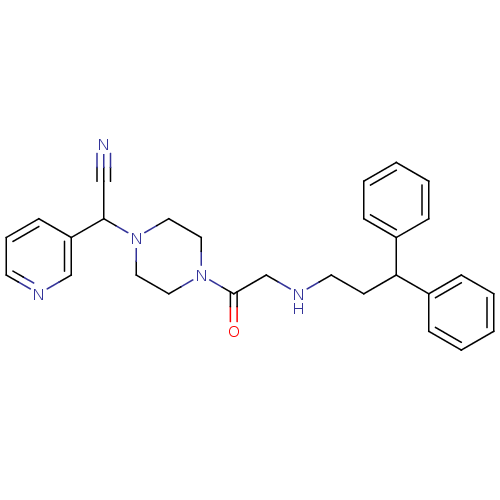
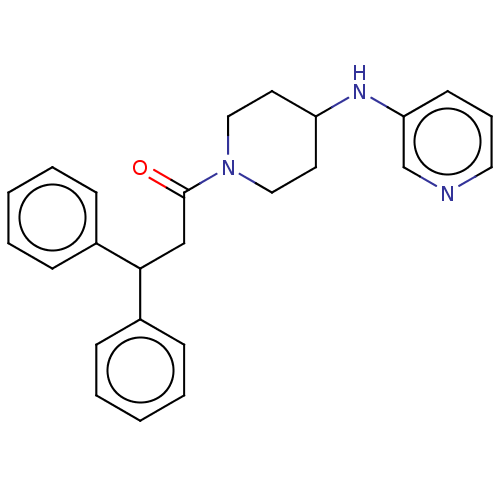
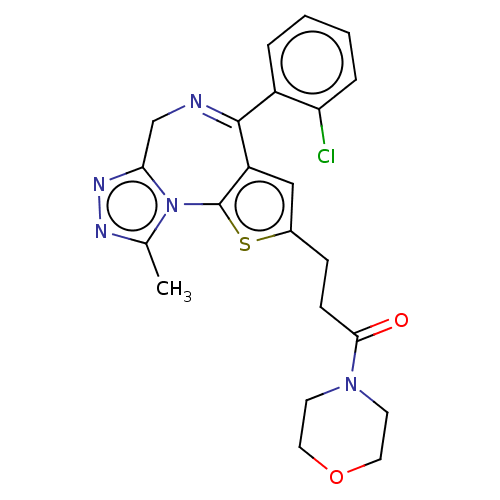
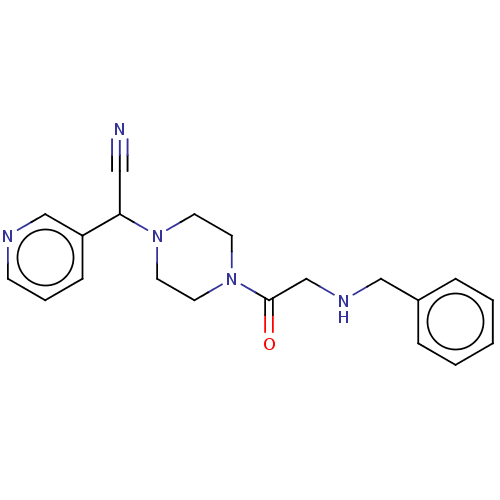
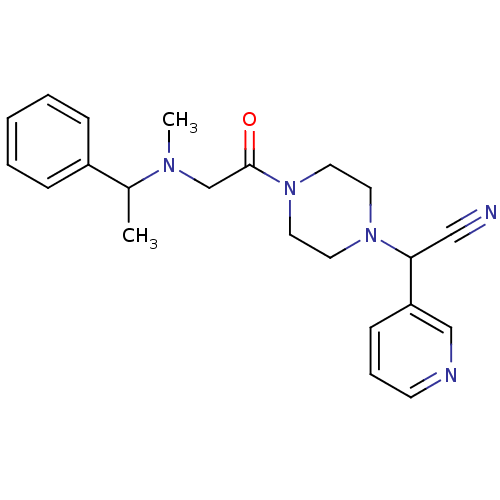
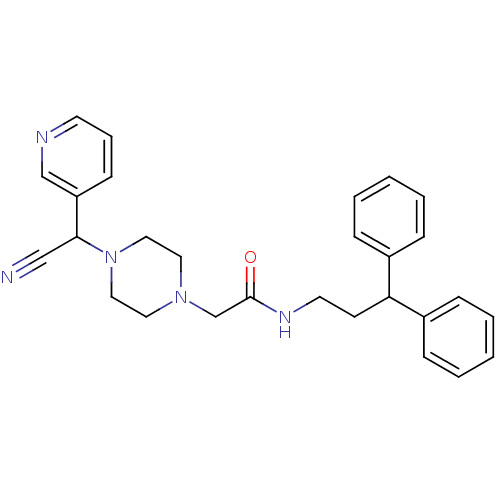
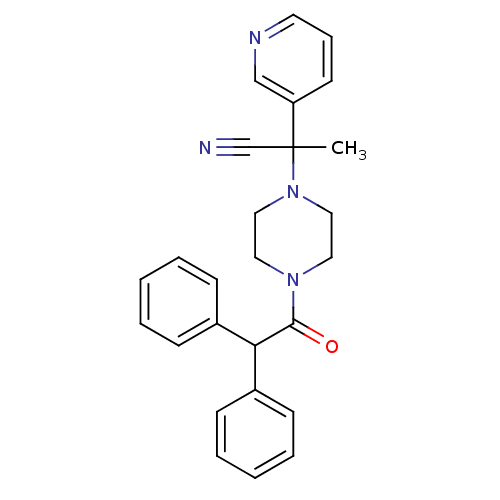
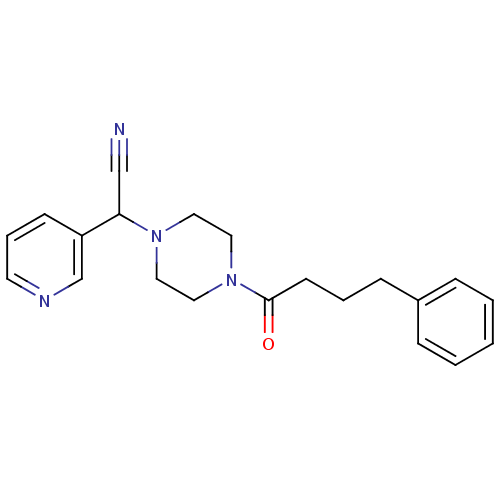
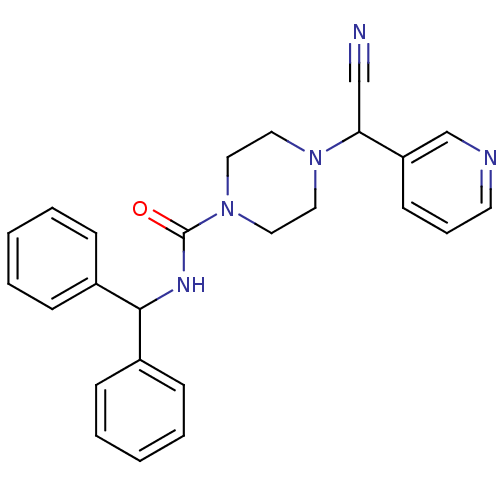
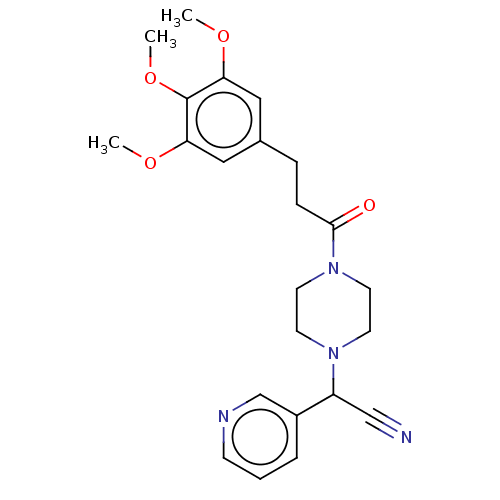
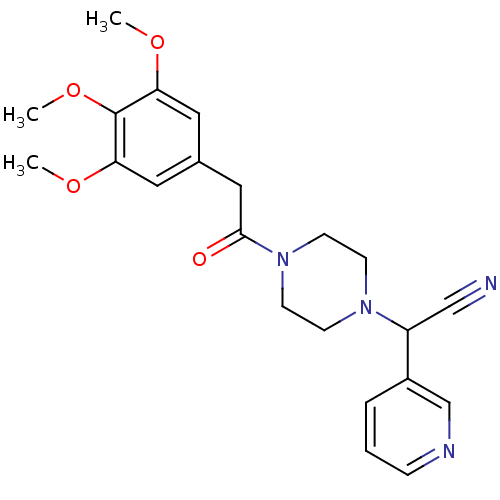
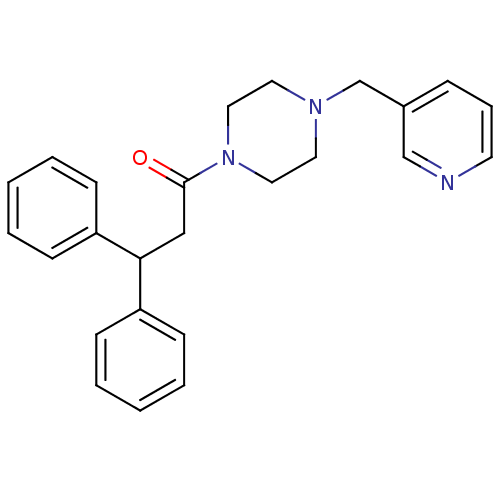
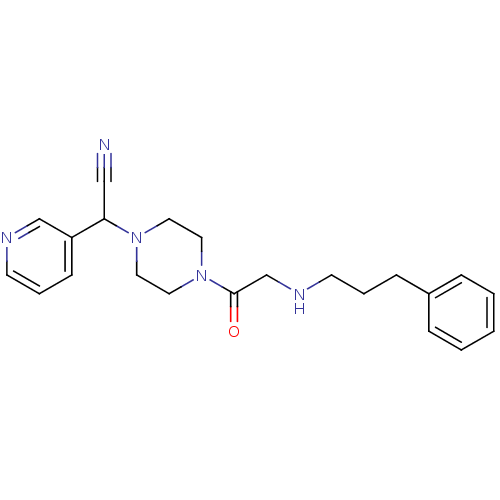
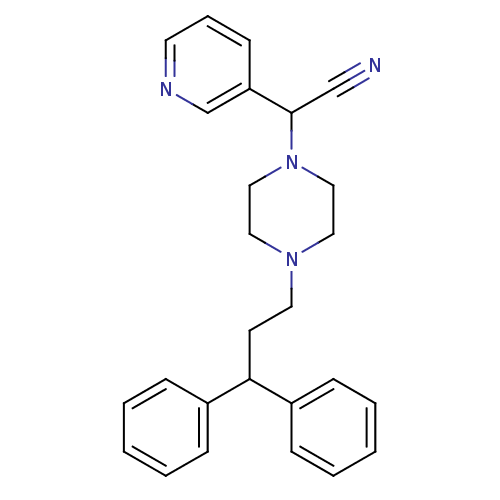
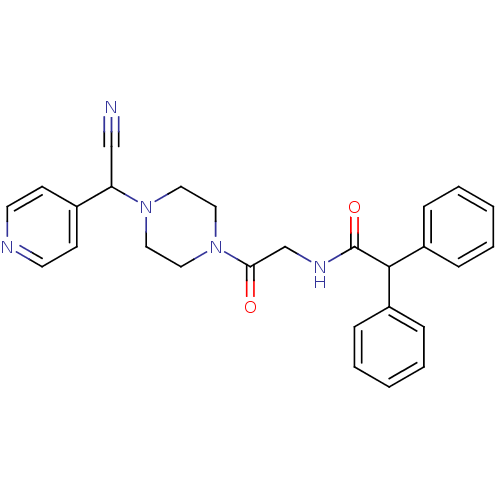
Affinity DataIC50: 1.90nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 7.5nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 9.10nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 53nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 76nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 86nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 87nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 91nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 230nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 240nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 240nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 240nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 240nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 260nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 360nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 450nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 460nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 520nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 520nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 530nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 630nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 680nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 720nMAssay Description:Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%.More data for this Ligand-Target Pair