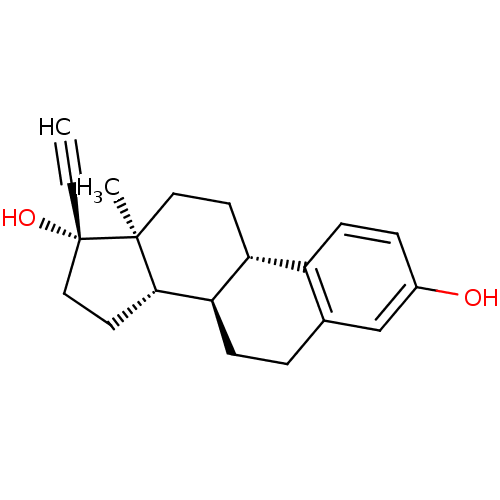
TargetEstrogen receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 0.610nMAssay Description:Binding affinity to ERalphaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:Binding affinity to ERbetaMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Binding affinity to ERalphaMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Binding affinity to ERalphaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 8.10nMAssay Description:Binding affinity to ERbetaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 8.20nMAssay Description:Binding affinity to ERbetaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 20.3nMAssay Description:Binding affinity to ERbetaMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 51nMAssay Description:Binding affinity to ERalphaMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Binding affinity to ERalphaMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Binding affinity to ERalphaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Binding affinity to ERbetaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Binding affinity to ERbetaMore data for this Ligand-Target Pair

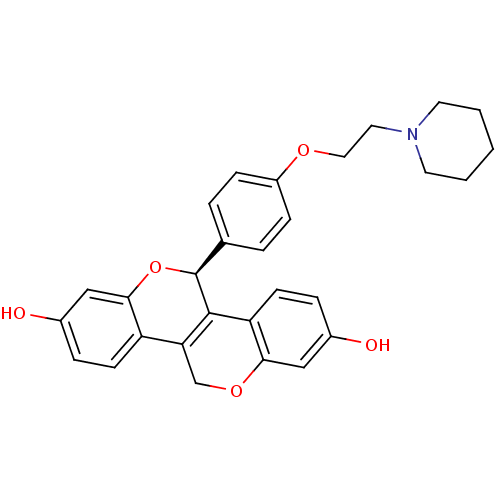
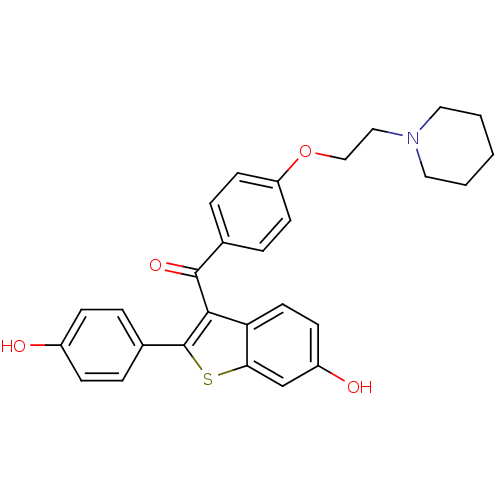
 3D Structure (crystal)
3D Structure (crystal)