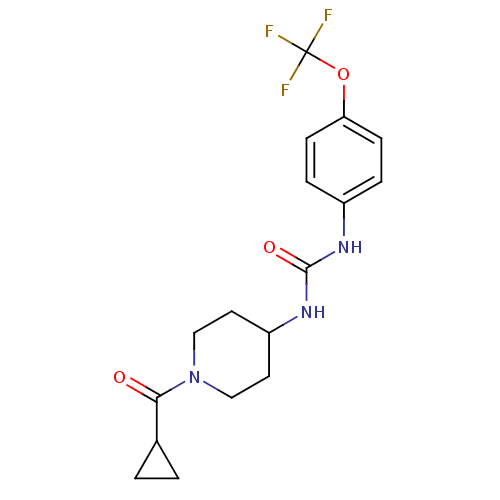
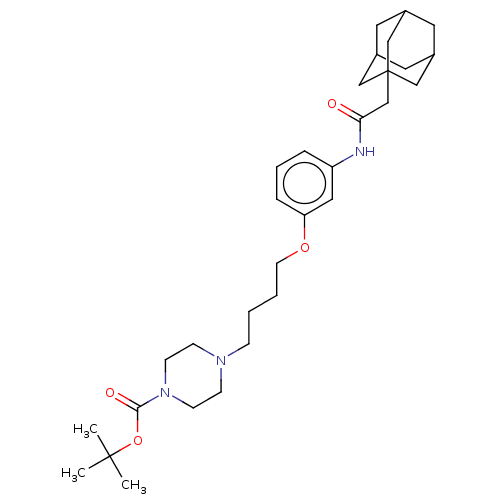
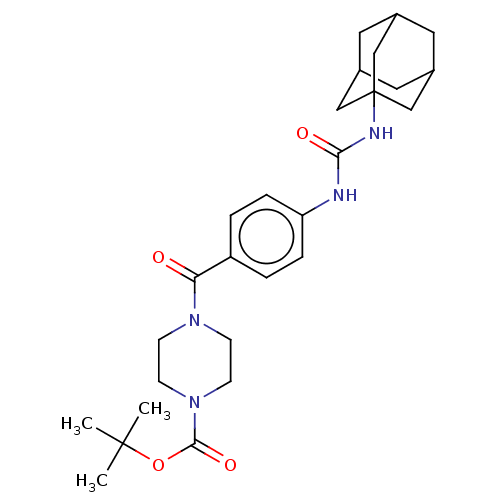
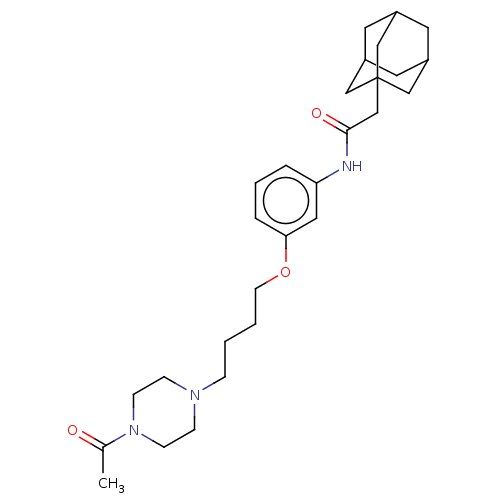
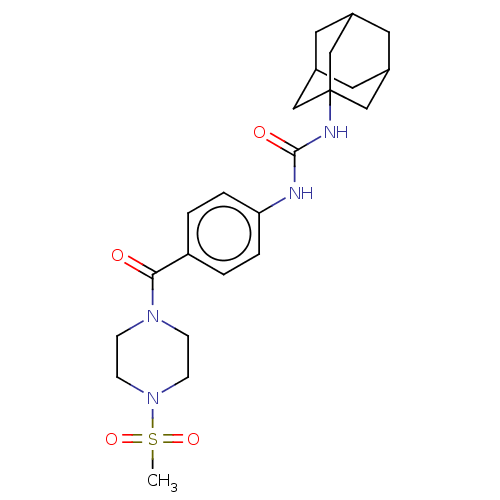
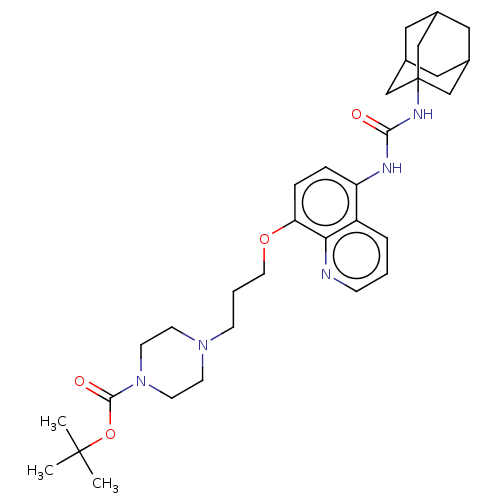
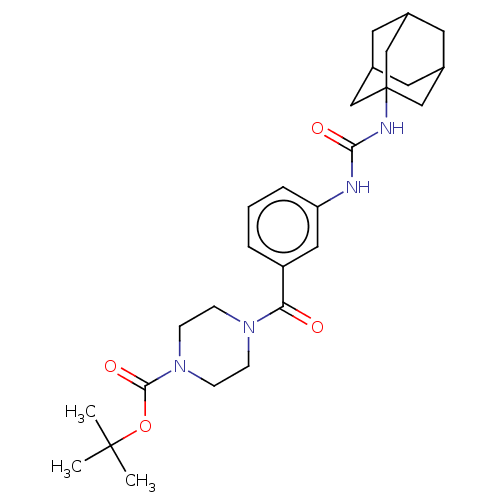
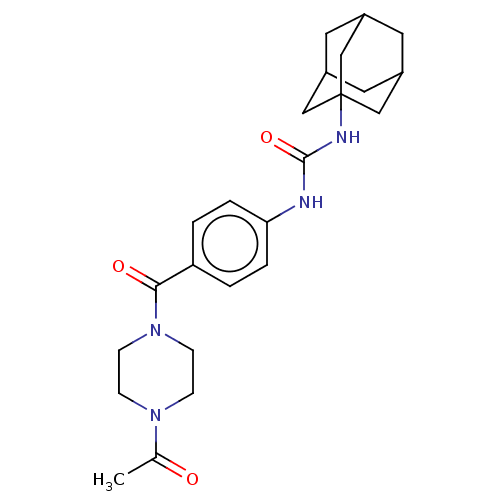
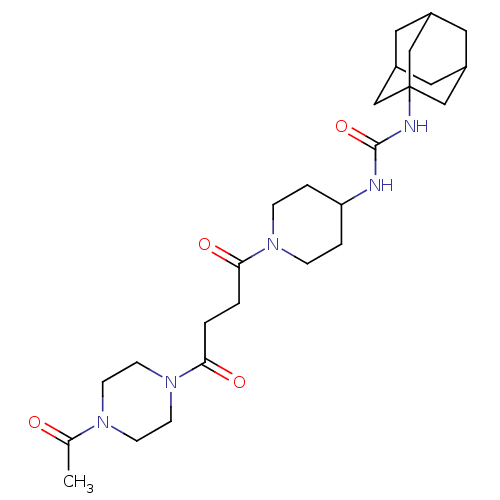
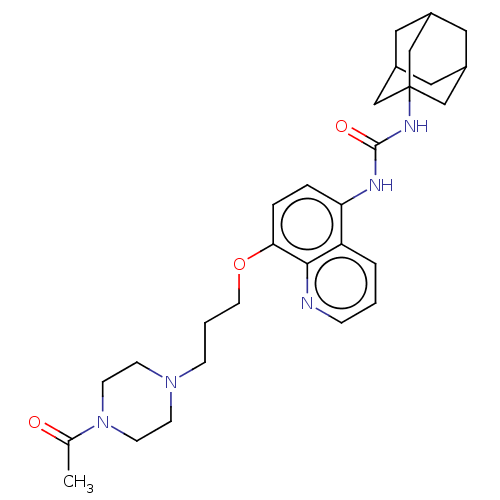
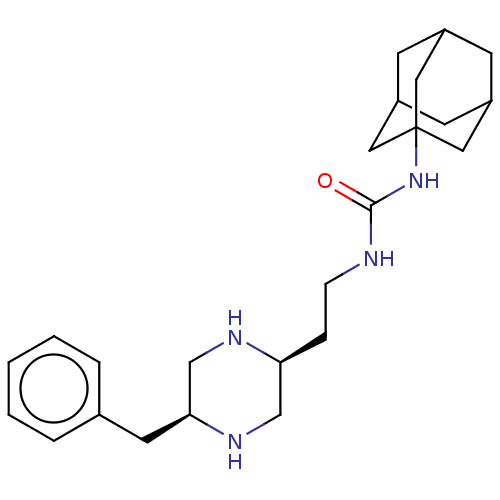
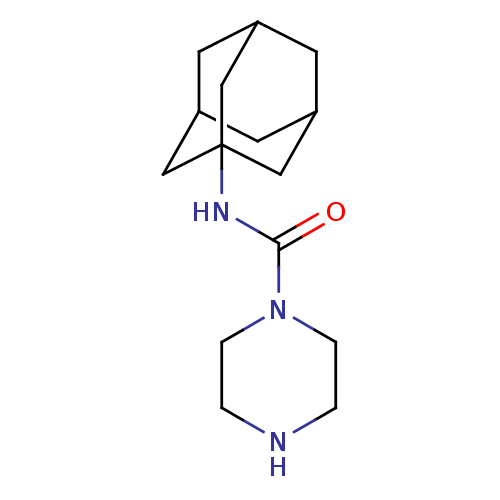
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of soluble epoxide hydrolase (unknown origin)More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 6.80nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 7.30nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 42nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 45nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 46nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 64nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 67nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 77nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 133nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 142nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 321nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 490nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.37E+3nMAssay Description:Inhibition of soluble epoxide hydrolase (unknown origin)More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair