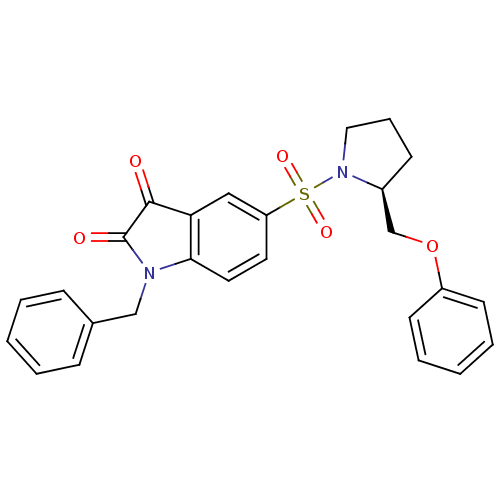
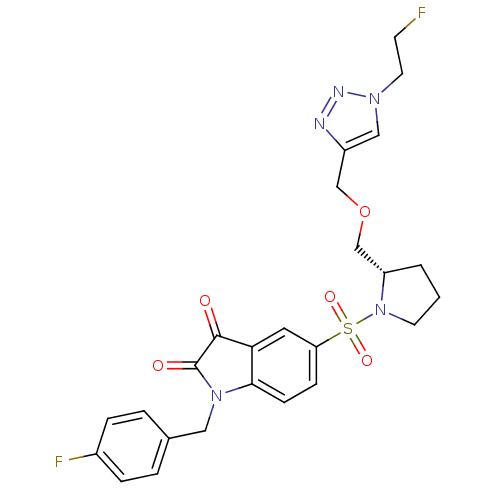
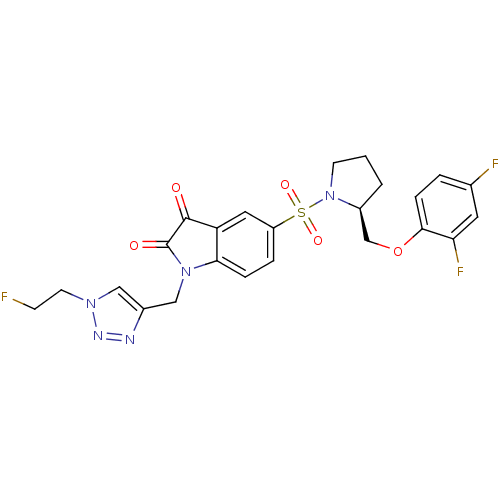
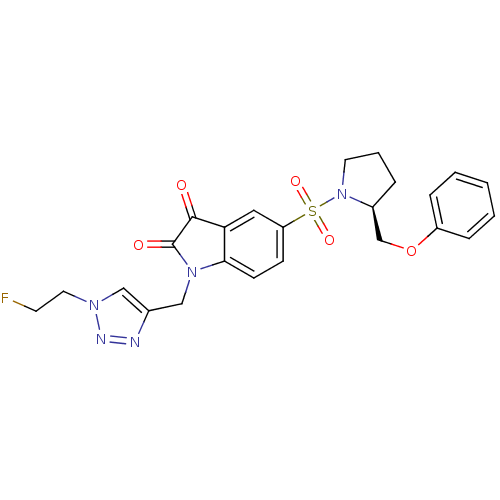
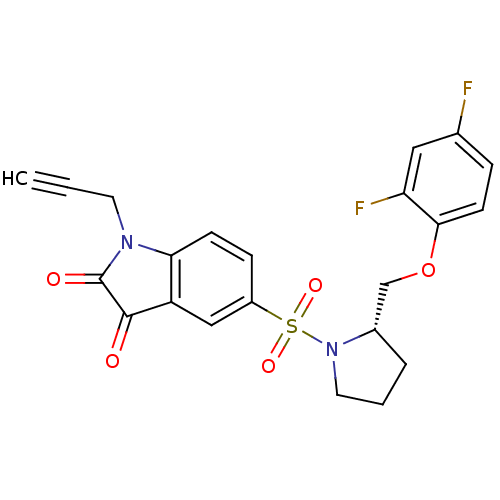
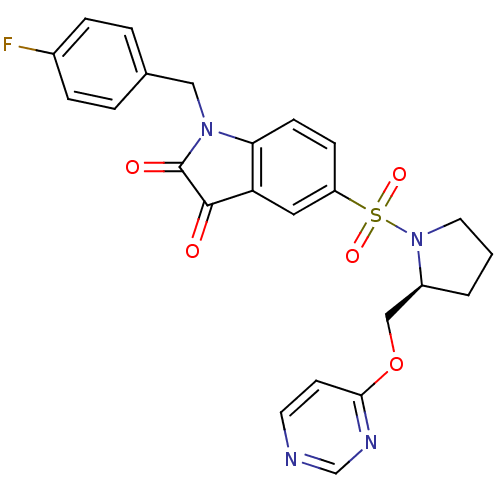
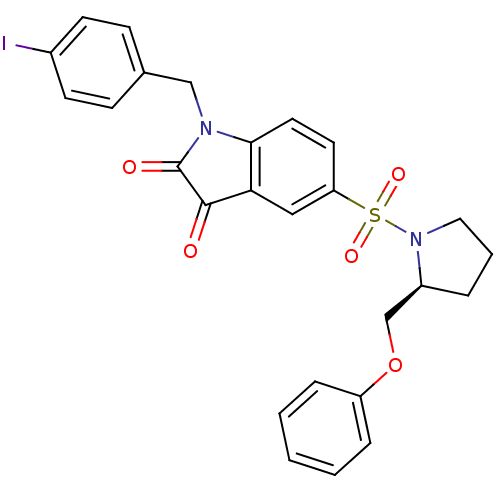
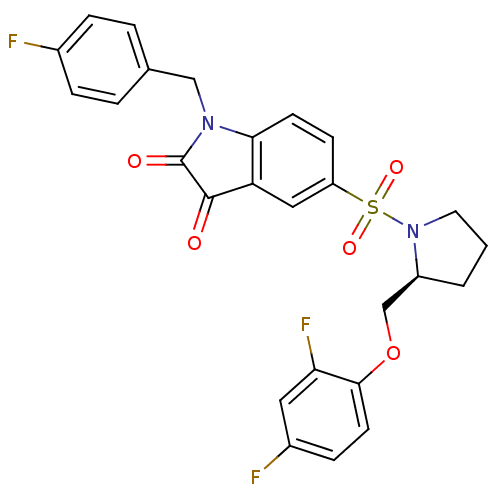
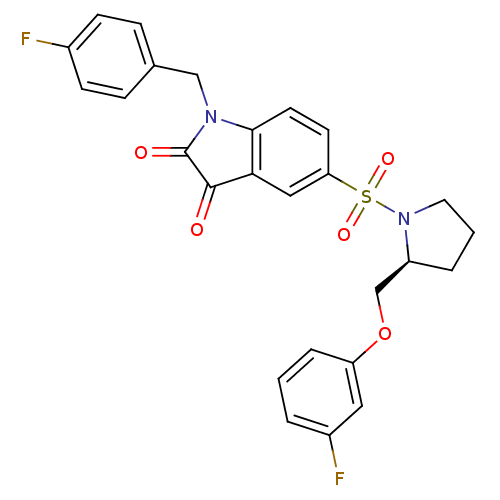
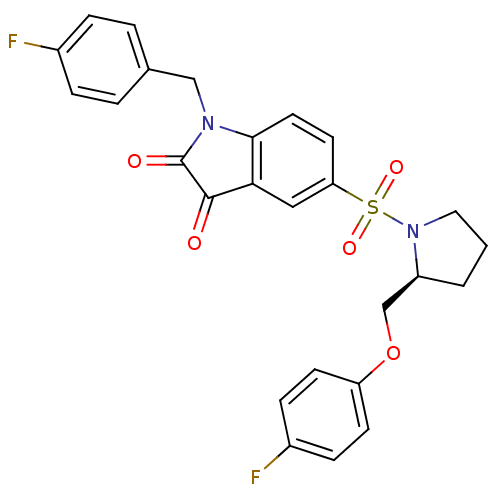
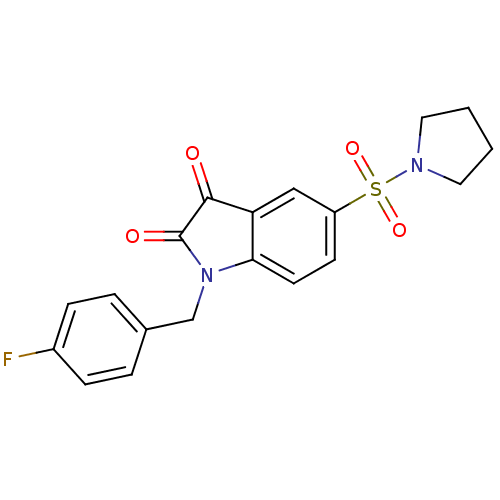
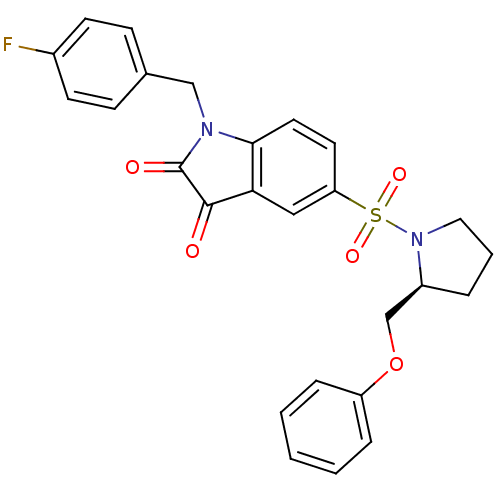
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair