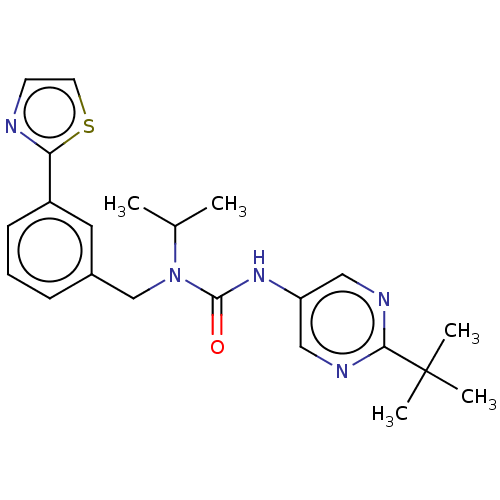
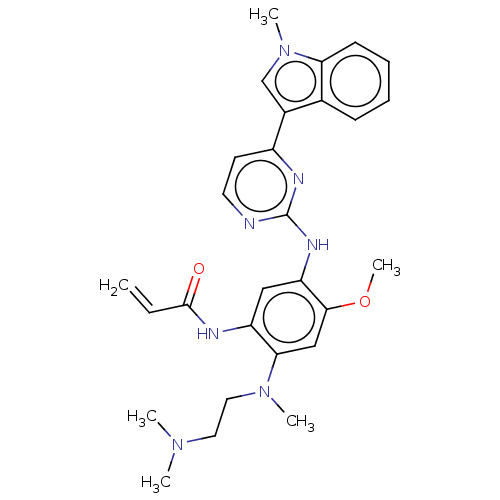
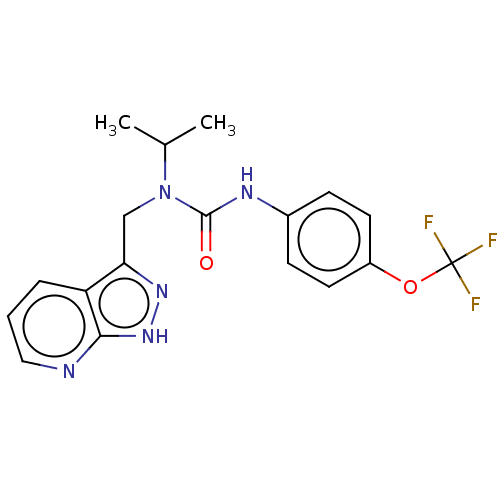
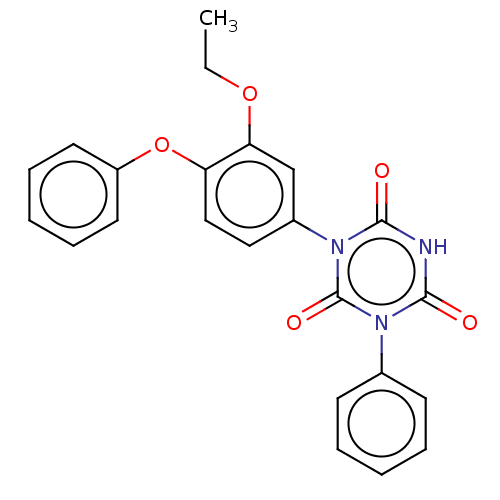
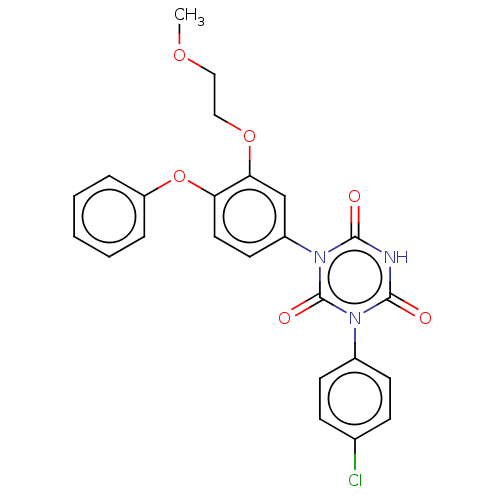
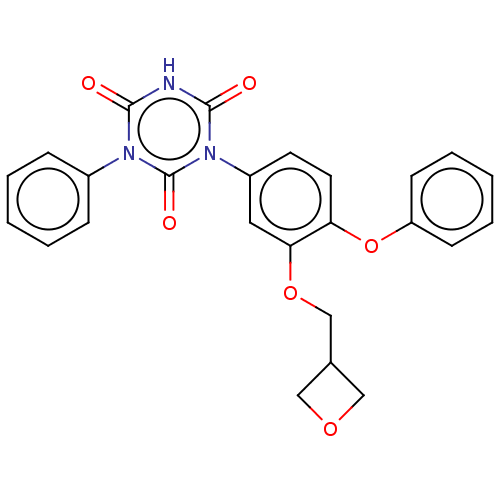
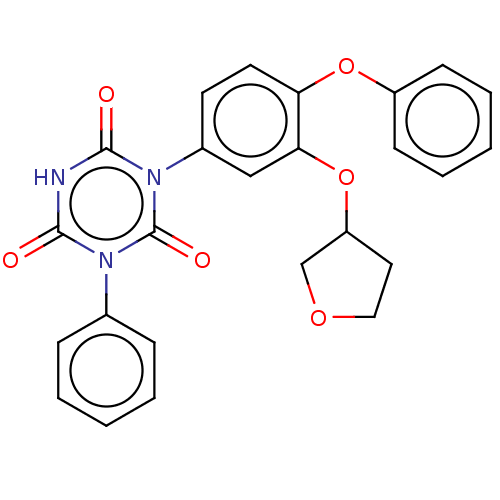
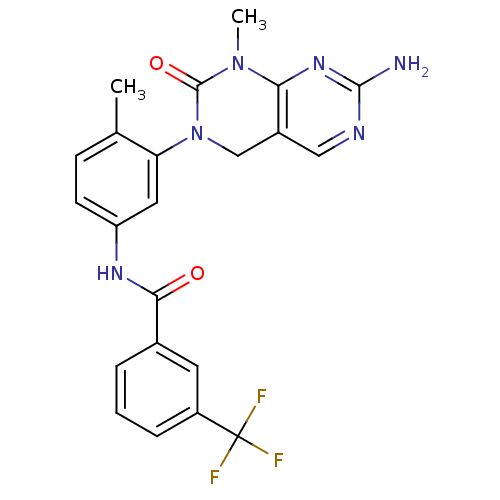
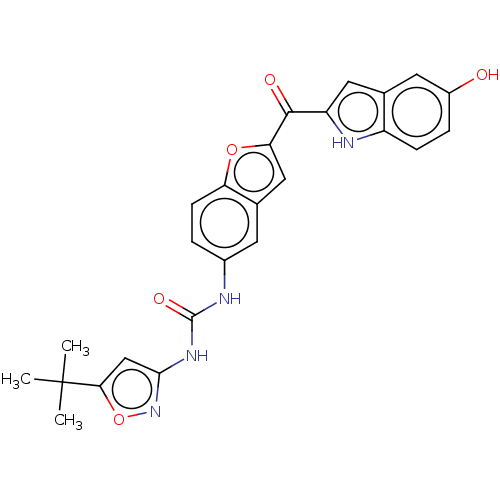
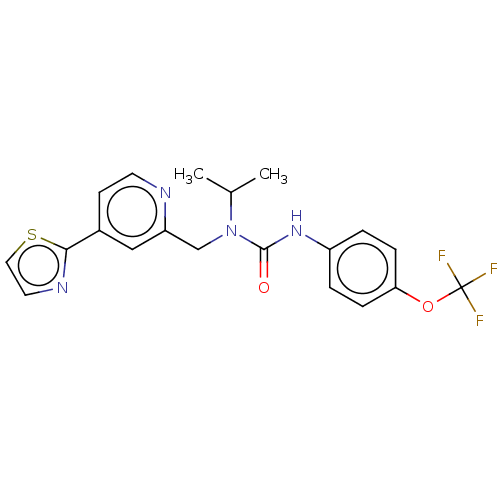
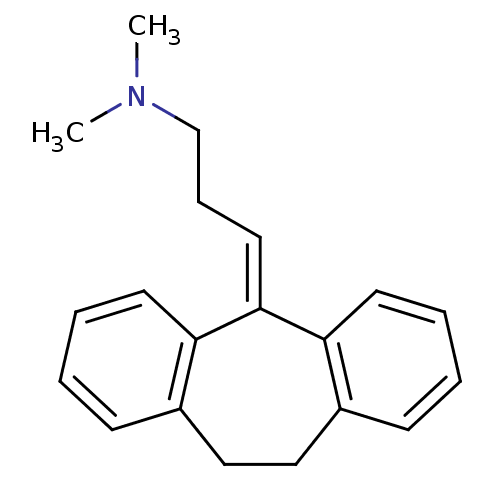
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataEC50: 2nMAssay Description:Inhibition of human Her2 in BT474 cellsMore data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataEC50: 2nMAssay Description:Inhibition of human Her2 in SKBR3 cellsMore data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataEC50: 3nMAssay Description:Inhibition of wild-type HER2 phosphorylation in human BT-474 cellsMore data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataEC50: 5nMAssay Description:Inhibition of HER2 phosphorylation in human BT-474 cells incubated for 3 hrs followed by replacement with fresh medium without compound measured afte...More data for this Ligand-Target Pair
Affinity DataEC50: 6.5nMT: 2°CAssay Description:TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an...More data for this Ligand-Target Pair
Affinity DataEC50: 6.5nMAssay Description:Inhibition of TrkA (unknown origin) using fluorescently labeled peptide substrate after 3 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 11nMAssay Description:Inhibition of TrkA (unknown origin) using fluorescently labeled peptide substrate after 3 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 11.3nMT: 2°CAssay Description:TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an...More data for this Ligand-Target Pair
Affinity DataEC50: 19.7nMT: 2°CAssay Description:TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an...More data for this Ligand-Target Pair
Affinity DataEC50: 24.9nMT: 2°CAssay Description:TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an...More data for this Ligand-Target Pair
Affinity DataEC50: 26nMAssay Description:Inhibition of TEL fused wild type TRKA (unknown origin) transfected in mouse BaF3 cells assessed as reduction in phosphorylation at Y490 residue afte...More data for this Ligand-Target Pair
Affinity DataEC50: <30nMAssay Description:Inhibition of TRKA in human KM-12-LUC cells assessed as reduction in AKT phosphorylation at S473 residue after 2 hrs by Western blot analysisMore data for this Ligand-Target Pair
Affinity DataEC50: <30nMAssay Description:Inhibition of TRKA in human KM-12-LUC cells assessed as reduction in AKT phosphorylation at T308 residue after 2 hrs by Western blot analysisMore data for this Ligand-Target Pair
Affinity DataEC50: <30nMAssay Description:Inhibition of TRKA in human KM-12-LUC cells assessed as reduction in ERK1/2 phosphorylation at T202/Y204 residues after 2 hrs by Western blot analysi...More data for this Ligand-Target Pair
Affinity DataEC50: 48nMT: 2°CAssay Description:TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an...More data for this Ligand-Target Pair
Affinity DataEC50: 61nMAssay Description:Binding affinity to human full length TRKA at inactive state expressed in rat PC-12 cells assessed as induction of kinase conformational change at 40...More data for this Ligand-Target Pair
Affinity DataEC50: 67nMAssay Description:Inhibition of LMNA fused TRKA G667C mutant (unknown origin) transfected in mouse BaF3 cells assessed as reduction in phosphorylation at Y490 residue ...More data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataEC50: 81nMAssay Description:Inhibition of human Her2 in A431 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 84nMAssay Description:Inhibition of TRKA (unknown origin) by cell-based assayMore data for this Ligand-Target Pair
Affinity DataEC50: 84nMAssay Description:Inhibition of TRKA (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 102nMT: 2°CAssay Description:TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an...More data for this Ligand-Target Pair
Affinity DataEC50: 119nMT: 2°CAssay Description:TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an...More data for this Ligand-Target Pair
Affinity DataEC50: 120nMAssay Description:Inhibition of LMNA fused TRKA G595R mutant (unknown origin) transfected in mouse BaF3 cells assessed as reduction in phosphorylation at Y490 residue ...More data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataEC50: 122nMAssay Description:Inhibition of wild-type HER2 phosphorylation in human BT-474 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 141nMT: 2°CAssay Description:TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an...More data for this Ligand-Target Pair
Affinity DataEC50: 180nMAssay Description:Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence...More data for this Ligand-Target Pair
Affinity DataEC50: 220nMAssay Description:Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence...More data for this Ligand-Target Pair
Affinity DataEC50: 220nMAssay Description:Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence...More data for this Ligand-Target Pair
Affinity DataEC50: 230nMAssay Description:Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence...More data for this Ligand-Target Pair
Affinity DataEC50: 290nMAssay Description:Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence...More data for this Ligand-Target Pair
Affinity DataEC50: 320nMAssay Description:Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence...More data for this Ligand-Target Pair
Affinity DataEC50: 340nMAssay Description:Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence...More data for this Ligand-Target Pair
Affinity DataEC50: 360nMAssay Description:Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence...More data for this Ligand-Target Pair
Affinity DataEC50: 390nMAssay Description:Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence...More data for this Ligand-Target Pair
Affinity DataEC50: 480nMAssay Description:Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence...More data for this Ligand-Target Pair
Affinity DataEC50: 500nMAssay Description:Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence...More data for this Ligand-Target Pair
Affinity DataEC50: 550nMAssay Description:Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence...More data for this Ligand-Target Pair
Affinity DataEC50: 667nMAssay Description:Agonist activity at RFP-fused TrkA receptor (unknown origin) expressed in HEK293T cells after 6 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataEC50: 690nMAssay Description:Inhibition of human Her2 in SW620 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 780nMAssay Description:Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence...More data for this Ligand-Target Pair
Affinity DataEC50: 830nMAssay Description:Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence...More data for this Ligand-Target Pair
Affinity DataEC50: 1.01E+3nMAssay Description:Inhibition of TrkA (unknown origin) using fluorescently labeled peptide substrate after 3 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 1.01E+3nMT: 2°CAssay Description:TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an...More data for this Ligand-Target Pair
Affinity DataEC50: 1.30E+3nMAssay Description:Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence...More data for this Ligand-Target Pair
Affinity DataEC50: >2.00E+3nMAssay Description:Inhibition of NTRK1More data for this Ligand-Target Pair
Affinity DataEC50: 3.26E+3nMAssay Description:Inhibition of human EPHA7 incubated for 30 mins by Kinobead based assayMore data for this Ligand-Target Pair
Affinity DataEC50: 3.86E+3nMAssay Description:Inhibition of human NTRK1 incubated for 30 mins by Kinobead based assayMore data for this Ligand-Target Pair
Affinity DataEC50: 8.08E+3nMT: 2°CAssay Description:TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an...More data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Binding affinity to human full length TRKA at active state expressed in rat PC-12 cells assessed as induction of kinase conformational change at 52 d...More data for this Ligand-Target Pair
Affinity DataEC50: 8.60E+4nMAssay Description:Antagonist activity at full length glycosylated human TrkA expressed in HEKN3S cells cells assessed as reduction in NGF-induced ERK 42/44 phosphoryla...More data for this Ligand-Target Pair

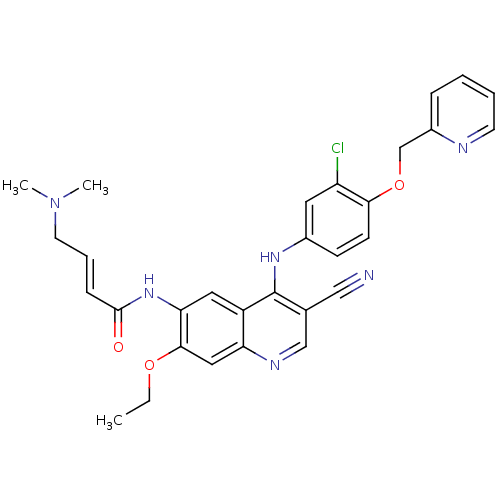

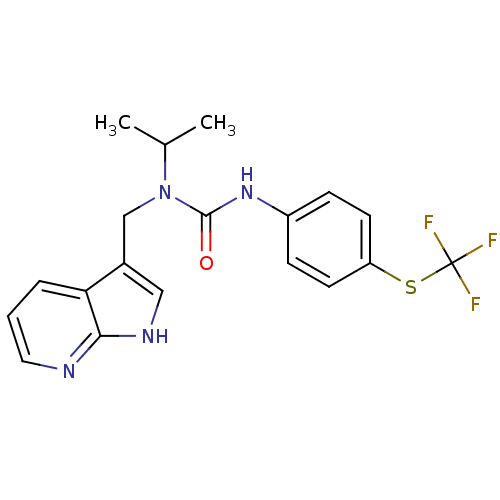


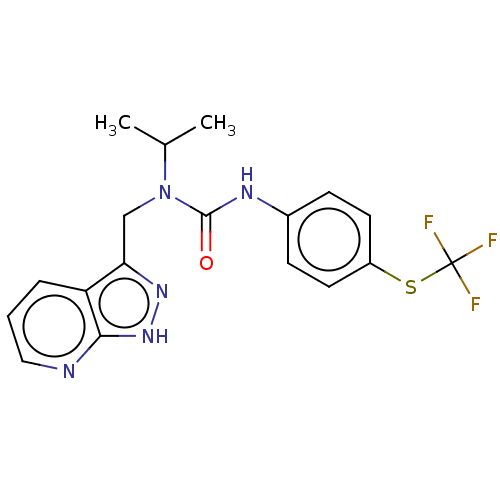
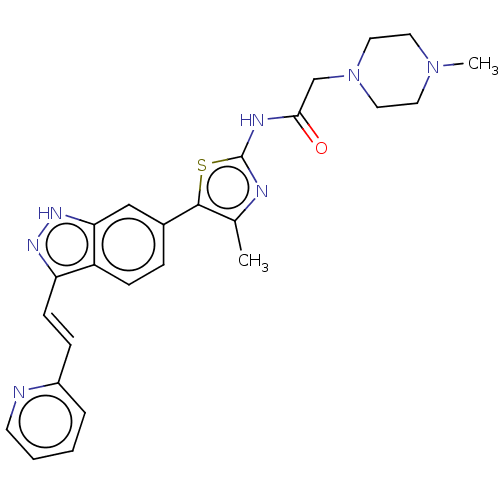


 3D Structure (crystal)
3D Structure (crystal)