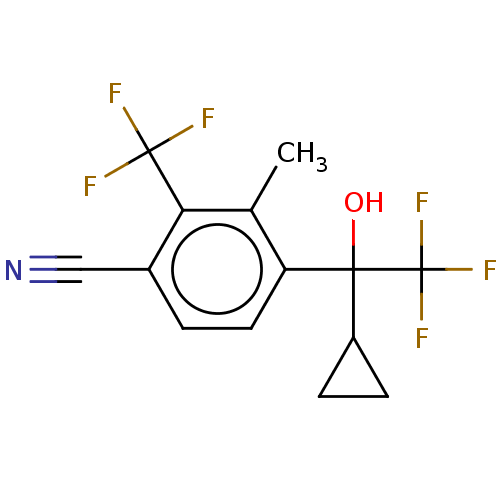
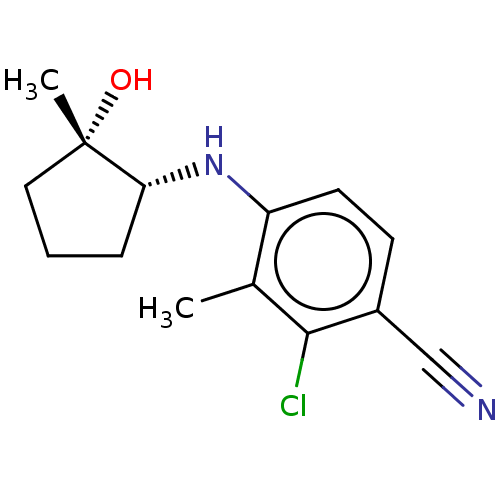
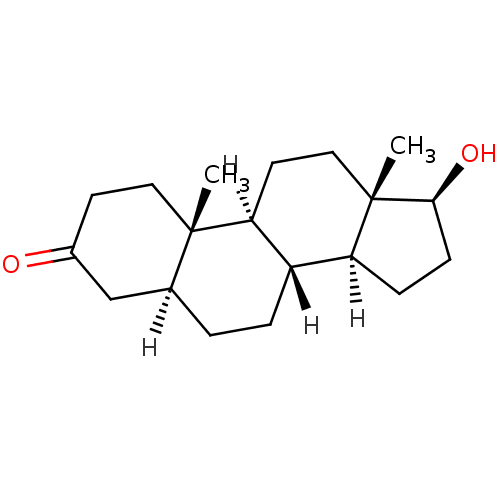
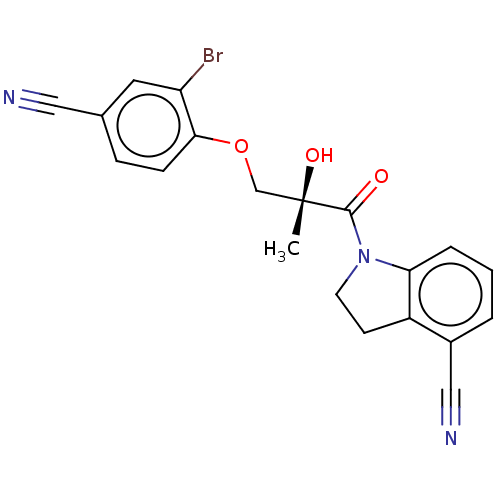
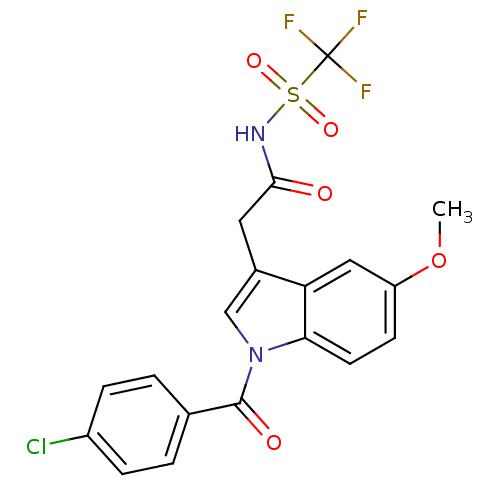
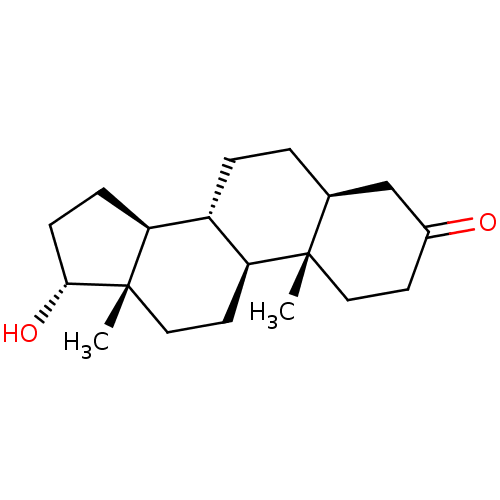
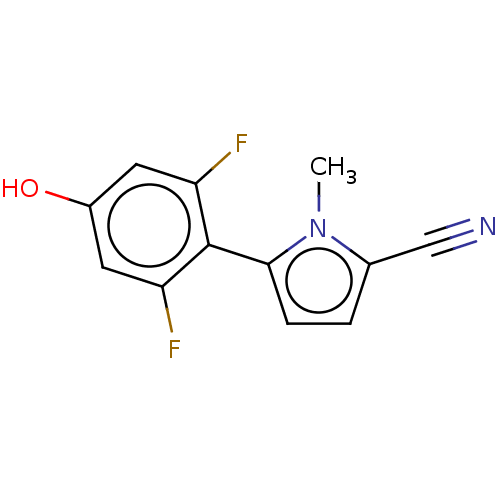
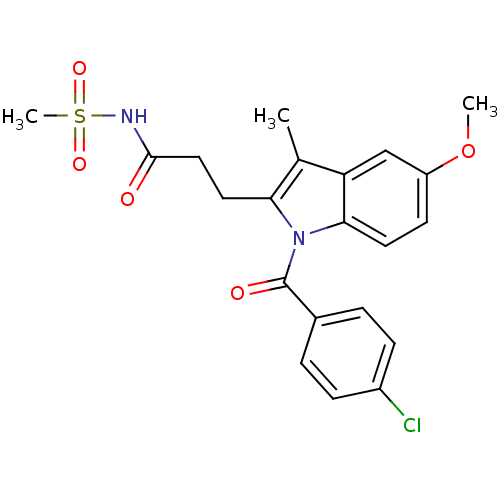
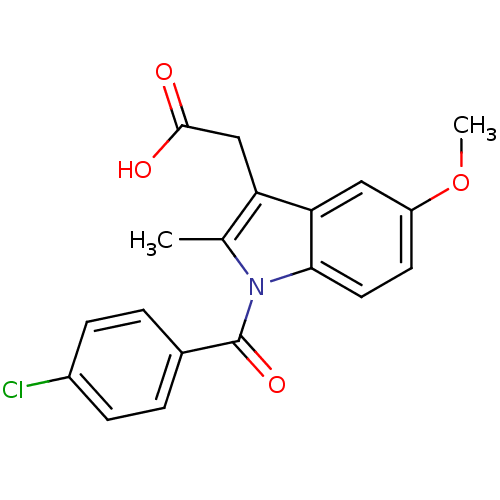
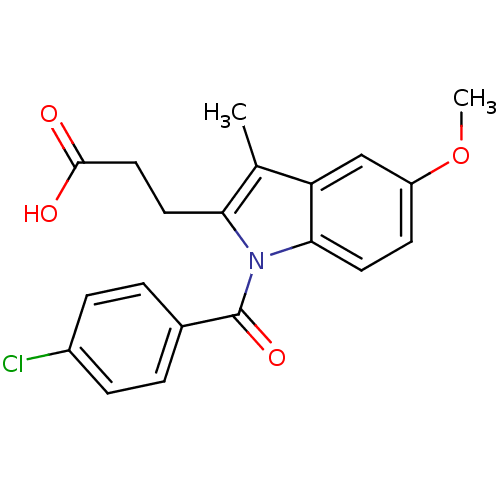
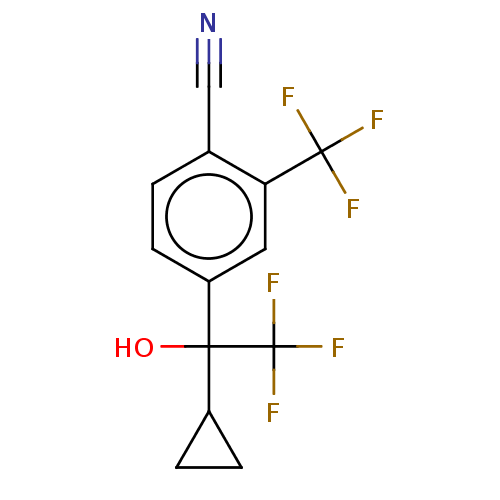
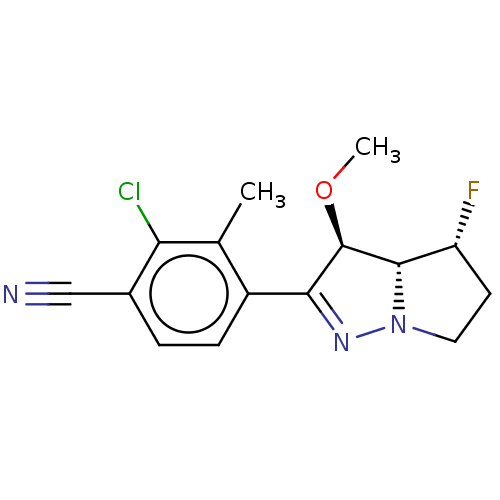
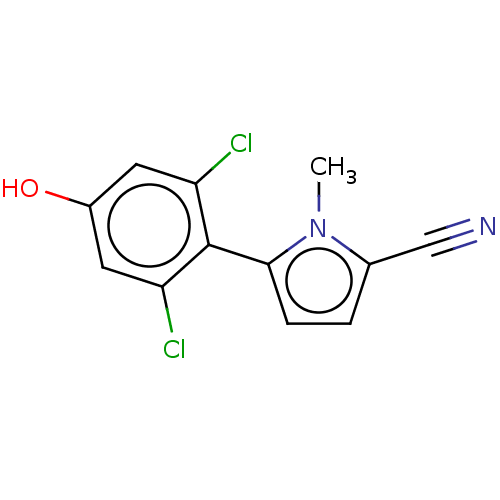
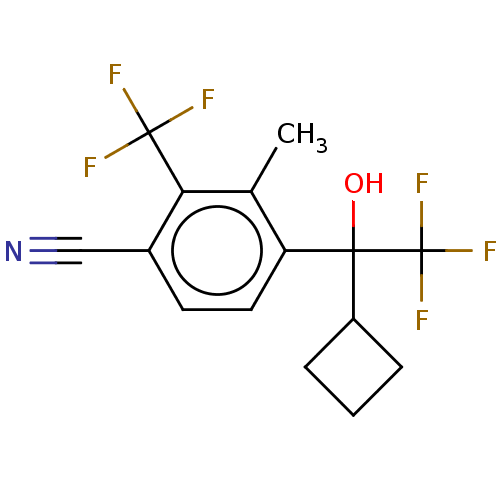
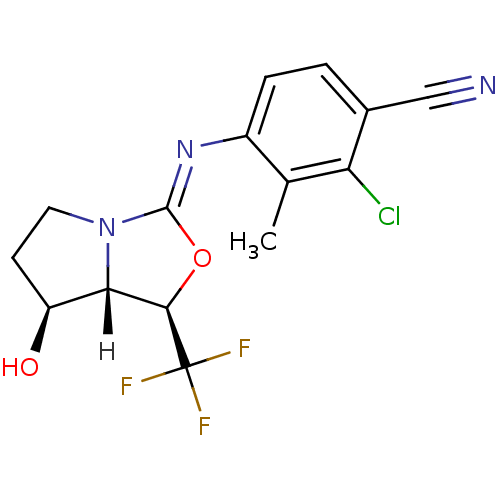
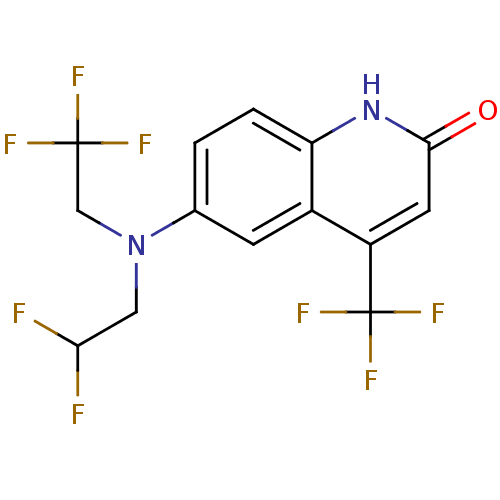
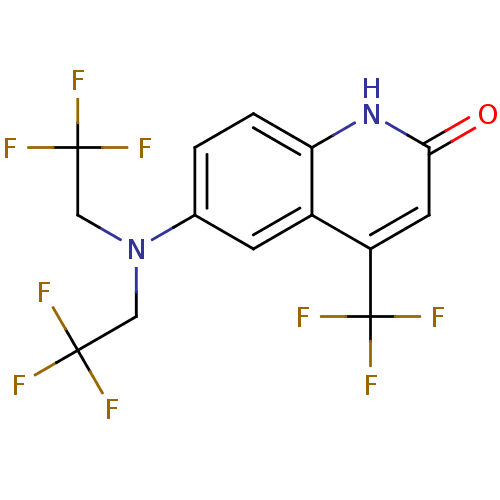
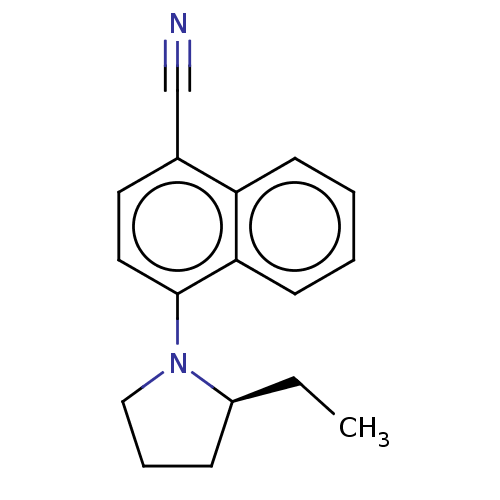
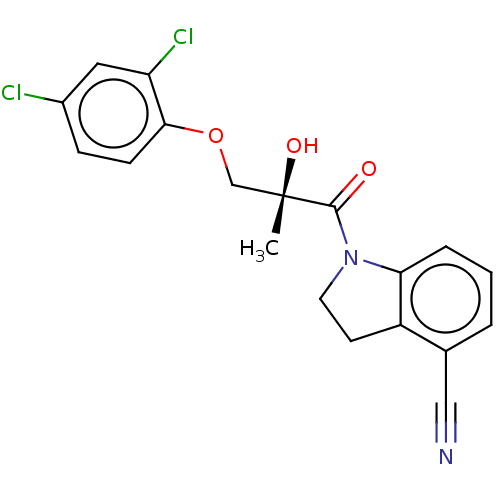
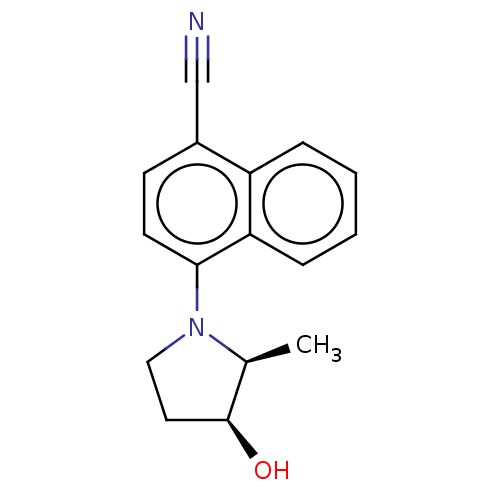
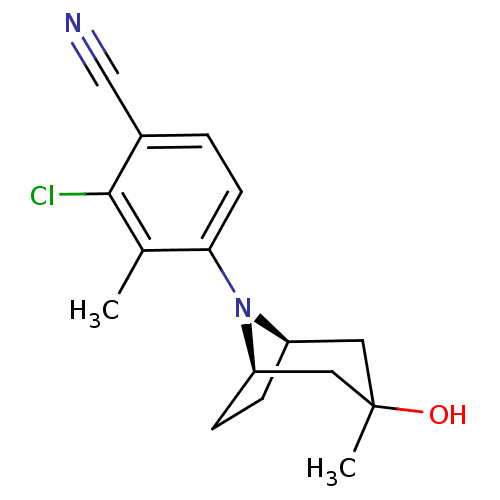
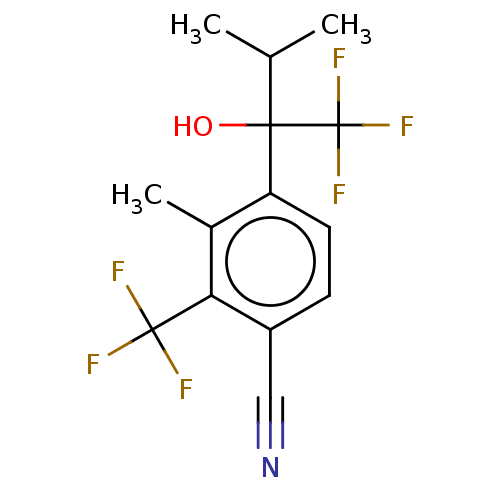
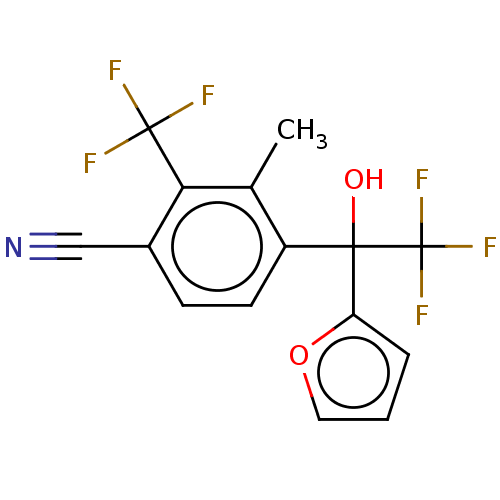
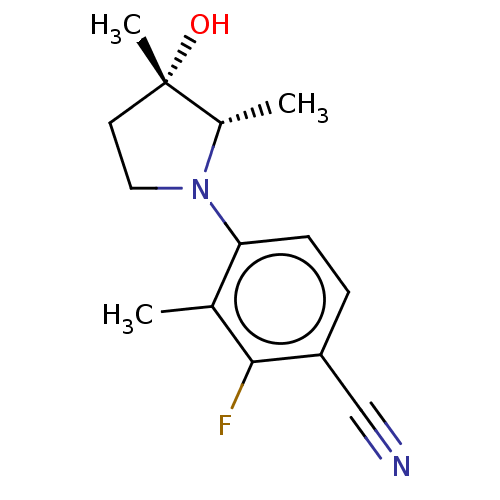
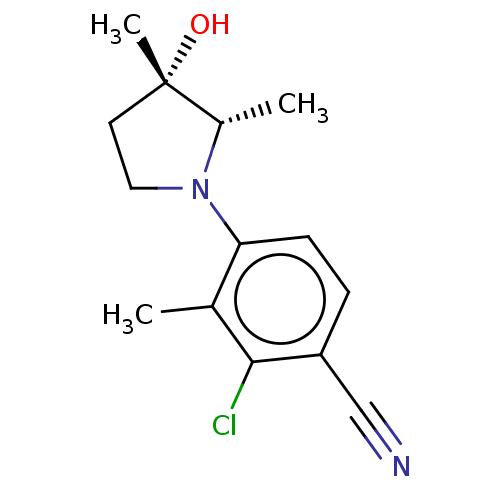
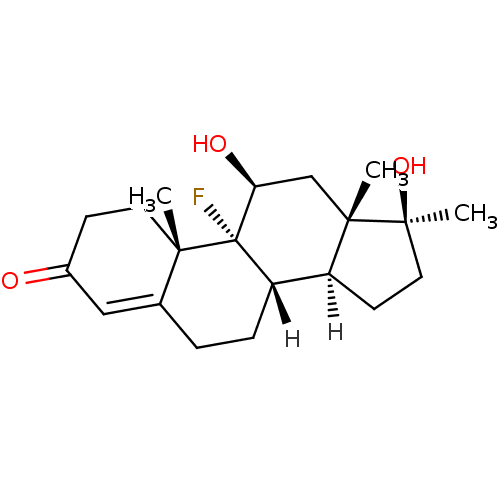
Affinity DataEC50: 0.0130nMAssay Description:Agonist activity at human androgen receptor expressed in mouse C2C12 cells cotransfected with GRE/ARE reporter plasmid assessed as receptor transacti...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0316nMAssay Description:Modulation of human androgen receptor expressed in African green monkey CV1 cells after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0390nMAssay Description:Agonist activity at human AR expressed in CV1 cells after 17 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0398nMAssay Description:Agonist activity at human androgen receptor expressed in mouse C2C12 cells cotransfected with GRE/ARE reporter plasmid assessed as receptor transacti...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0500nMAssay Description:Agonist activity at androgen receptor expressed in mouse C2C12 cells assessed as osteoblast differentiation after 5 daysMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0600nMAssay Description:Agonist activity at human AR expressed in CV1 cells after 17 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0740nMAssay Description:Agonist activity at human AR expressed in CV1 cells after 17 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.100nMAssay Description:Agonist activity at human Androgen receptor measured by CALUX bioassayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.100nMAssay Description:Agonist activity at androgen receptor (unknown origin) expressed in HeLa cells co-expressing PSA-(ARE)4-Luc13 assessed as induction of DHT-induced lu...More data for this Ligand-Target Pair
Affinity DataEC50: 0.100nMAssay Description:Agonist activity at androgen receptor expressed in mouse C2C12 cells assessed as osteoblast differentiation after 5 daysMore data for this Ligand-Target Pair
Affinity DataEC50: 0.110nMAssay Description:Agonist activity at human AR expressed in CV1 cells after 17 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.110nMAssay Description:Modulation of Androgen receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 0.130nMAssay Description:Agonist activity at human androgen receptor expressed in mouse C2C12 cells by androgen-specific response element-driven luciferase reporter gene assa...More data for this Ligand-Target Pair
Affinity DataEC50: 0.140nMAssay Description:Modulation of Androgen receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 0.140nMAssay Description:Agonist activity at human androgen receptor expressed in African green monkey CV-1 cells after 17 hrs by ARE luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.140nMAssay Description:Agonist activity at androgen receptor (unknown origin) expressed in HeLa cells co-expressing PSA-(ARE)4-Luc13 assessed as induction of DHT-induced lu...More data for this Ligand-Target Pair
Affinity DataEC50: 0.140nMAssay Description:Agonist activity at androgen receptor (unknown origin) expressed in HeLa cells co-expressing PSA-(ARE)4-Luc13 assessed as induction of DHT-induced lu...More data for this Ligand-Target Pair
Affinity DataEC50: 0.140nMAssay Description:Binding affinity to human AR NTD (1 to 561 aa) expressed in CV1 cells coexpressing human AR-LBD (644-919 aa) assessed as N/C-termini interaction by a...More data for this Ligand-Target Pair
Affinity DataEC50: 0.150nMAssay Description:Agonist activity at human AR expressed in CV1 cells after 17 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.150nMAssay Description:Agonist activity at androgen receptor (unknown origin) expressed in HeLa cells co-expressing PSA-(ARE)4-Luc13 assessed as induction of DHT-induced lu...More data for this Ligand-Target Pair
Affinity DataEC50: 0.158nMAssay Description:Modulation of human androgen receptor expressed in African green monkey CV1 cells after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.160nMAssay Description:Agonist activity at human androgen receptor expressed in mouse C2C12 cells by androgen-specific response element-driven luciferase reporter gene assa...More data for this Ligand-Target Pair
Affinity DataEC50: 0.170nMAssay Description:Agonist activity at human androgen receptor expressed in African green monkey CV-1 cells after 17 hrs by ARE luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.180nMAssay Description:Agonist activity at human androgen receptor expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.190nMAssay Description:Agonist activity at human AR expressed in African green monkey COS7 cells after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.190nMAssay Description:Modulation of Androgen receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 0.190nMAssay Description:Modulation of androgen receptor (unknown origin) expressed in Cos7 cells assessed as luciferase activity after 24 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Modulation of human androgen receptor expressed in African green monkey CV1 cells after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Modulation of human androgen receptor expressed in African green monkey CV1 cells after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Modulation of human androgen receptor expressed in African green monkey CV1 cells after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nM ΔG°: -53.8kJ/mole EC50: 0.200nMpH: 7.6 T: 2°CAssay Description:Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB...More data for this Ligand-Target Pair
Affinity DataKi: 1.70nM ΔG°: -52.1kJ/mole EC50: 0.200nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 4.60nM ΔG°: -49.5kJ/mole EC50: 0.200nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at human Androgen receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Modulation of Androgen receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at human Androgen receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.240nMAssay Description:Modulation of androgen receptor (unknown origin) expressed in Cos7 cells assessed as luciferase activity after 24 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.25nMAssay Description:Agonist activity at human AR expressed in CV1 cells after 17 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.25nMAssay Description:Modulation of androgen receptor (unknown origin) expressed in Cos7 cells assessed as luciferase activity after 24 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.251nMAssay Description:Agonist activity at androgen receptor in human MDA-KB2 cells transfected with MMTV linked luciferase assessed as transcriptional activation by lucife...More data for this Ligand-Target Pair
Affinity DataEC50: 0.251nMAssay Description:Modulation of human androgen receptor expressed in African green monkey CV1 cells after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.251nMAssay Description:Modulation of human androgen receptor expressed in African green monkey CV1 cells after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.280nMAssay Description:Agonist activity at human AR expressed in CV1 cells after 17 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.290nMAssay Description:Agonist activity at human AR expressed in African green monkey COS7 cells after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.290nMAssay Description:Modulation of Androgen receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 0.290nMAssay Description:Agonist activity to human Androgen receptor in African green monkey COS-7 cells measured by luciferase reporter assayMore data for this Ligand-Target Pair
Affinity DataKi: 5.70nM ΔG°: -48.9kJ/mole EC50: 0.300nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataEC50: 0.300nMAssay Description:Agonist activity at human androgen receptor expressed in mouse C2C12 cells by androgen-specific response element-driven luciferase reporter gene assa...More data for this Ligand-Target Pair
Affinity DataEC50: 0.300nMAssay Description:Transcriptional repression in HepG2 cells expressing human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.320nMAssay Description:Agonist activity to human Androgen receptor in African green monkey COS-7 cells measured by luciferase reporter assayMore data for this Ligand-Target Pair

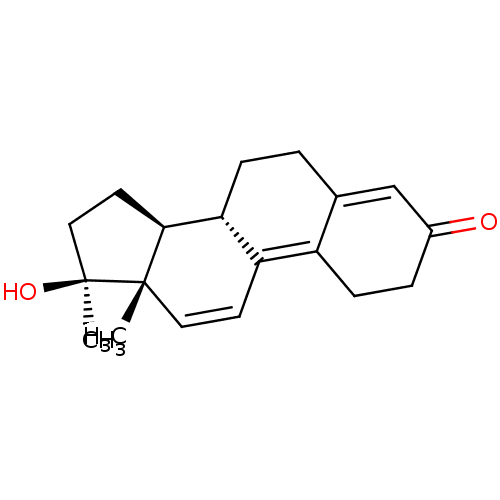
 3D Structure (crystal)
3D Structure (crystal)