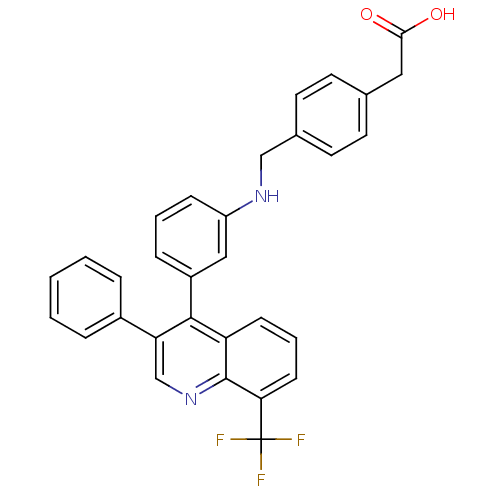
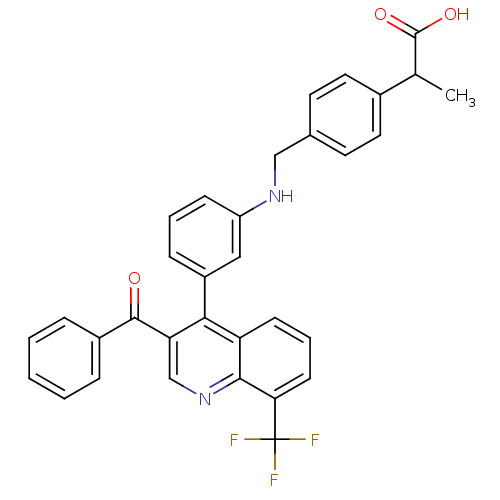
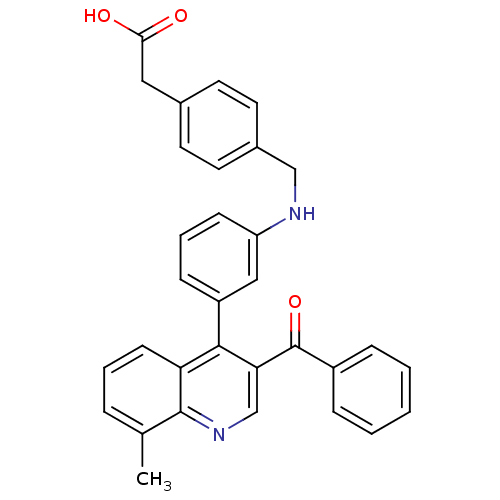
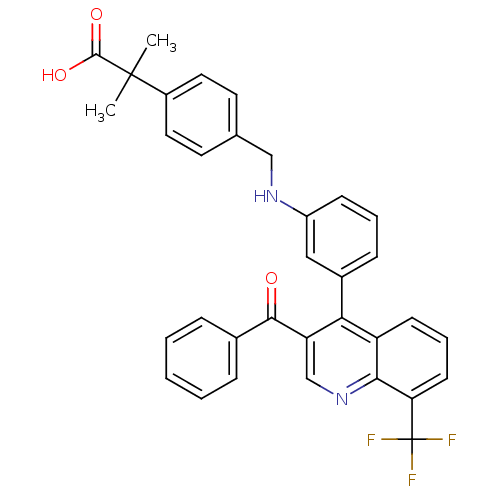
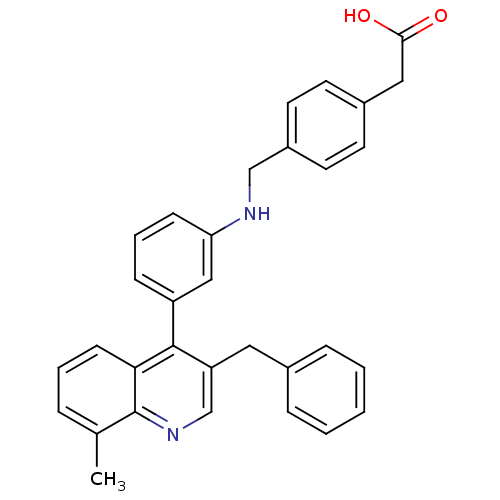
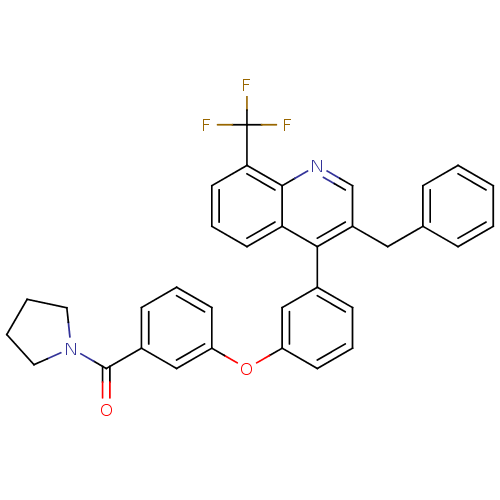
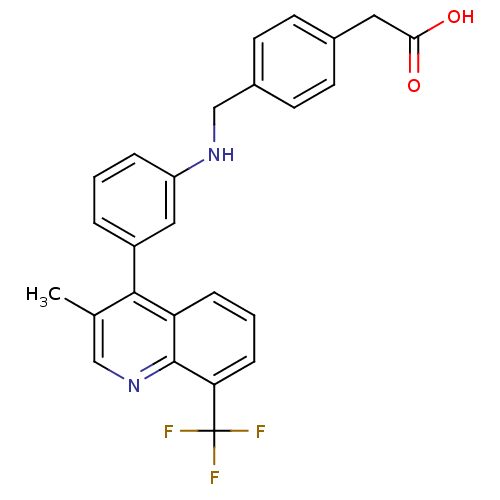
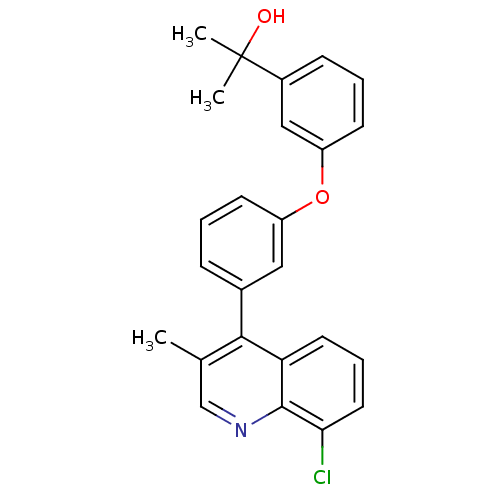
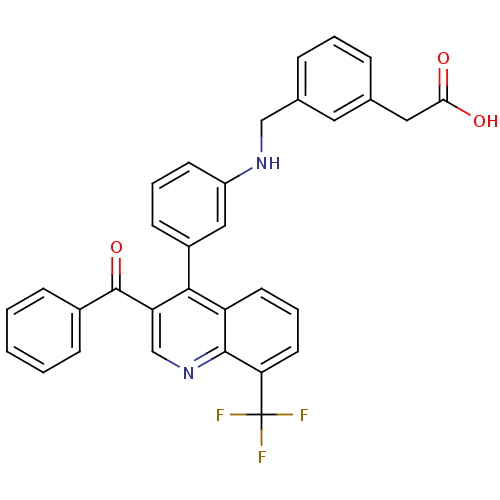
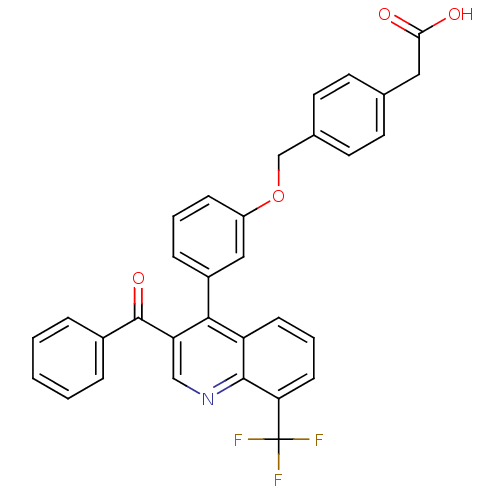
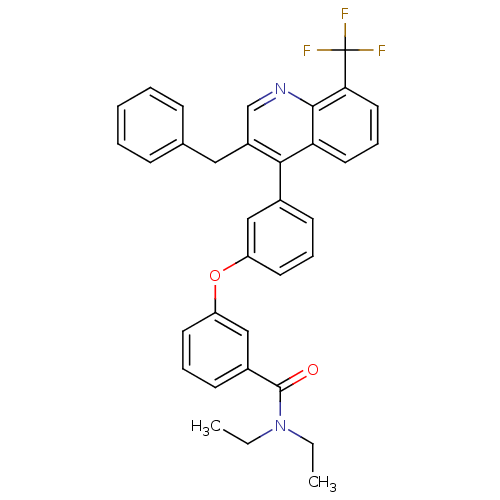
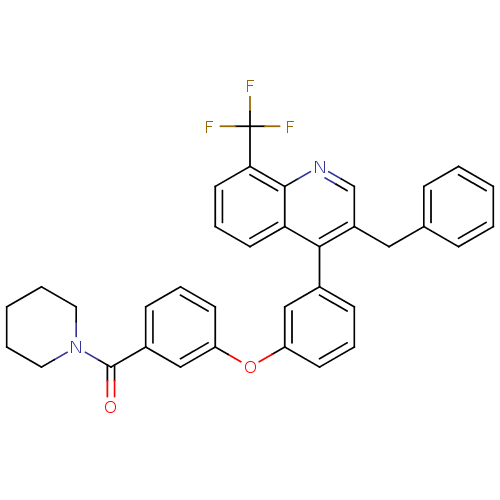
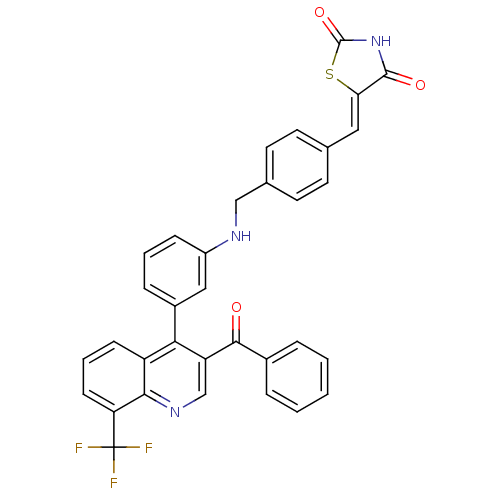
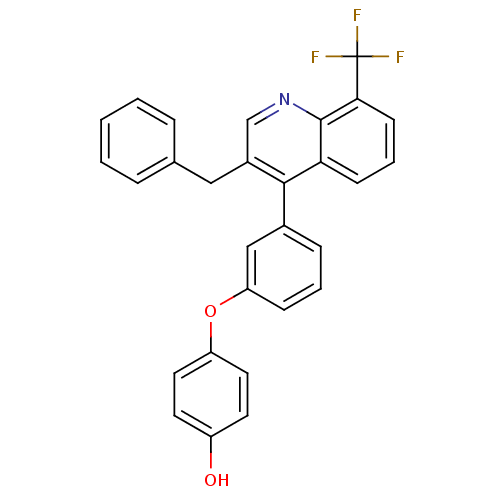
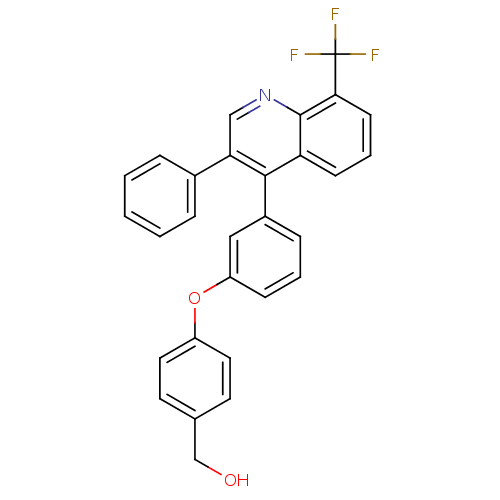
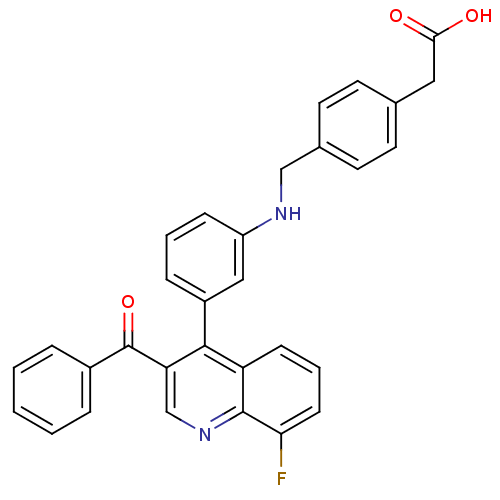
Affinity DataIC50: 10nM EC50: 16nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 9nM EC50: 16nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nM EC50: 23nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nM EC50: 29nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 3.30nM EC50: 31nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nM EC50: 33nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nM EC50: 33nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70nM EC50: 39nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 3.70nM EC50: 44nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nM EC50: 58nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nM EC50: 61nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nM EC50: 71nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nM EC50: 71nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80nM EC50: 78nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 5nM EC50: 90nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 2nM EC50: 90nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 2nM EC50: 90nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nM EC50: 93nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nM EC50: 98nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 4.30nM EC50: 100nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nM EC50: 108nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nM EC50: 138nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 6nM EC50: 141nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 5nM EC50: 143nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nM EC50: 144nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nM EC50: 170nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 9nM EC50: 170nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 67nM EC50: 186nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 14nM EC50: 210nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nM EC50: 233nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ...More data for this Ligand-Target Pair
Affinity DataIC50: 310nM EC50: 286nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nM EC50: 296nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 12nM EC50: 310nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nM EC50: 316nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 138nM EC50: 365nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 276nM EC50: 369nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 115nM EC50: 374nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 28nM EC50: 406nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ...More data for this Ligand-Target Pair
Affinity DataIC50: 12nM EC50: 410nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 33.8nM EC50: 450nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 7nM EC50: 572nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 287nM EC50: 612nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ...More data for this Ligand-Target Pair
Affinity DataIC50: 10nM EC50: 646nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ...More data for this Ligand-Target Pair
Affinity DataIC50: 17nM EC50: 700nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 45nM EC50: 770nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 5.40nM EC50: 795nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 9nM EC50: 800nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 56.4nM EC50: 812nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 53nM EC50: 895nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ...More data for this Ligand-Target Pair
Affinity DataIC50: 12nM EC50: 938nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)