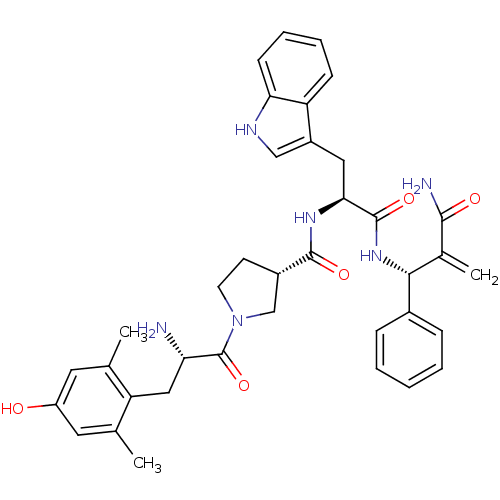
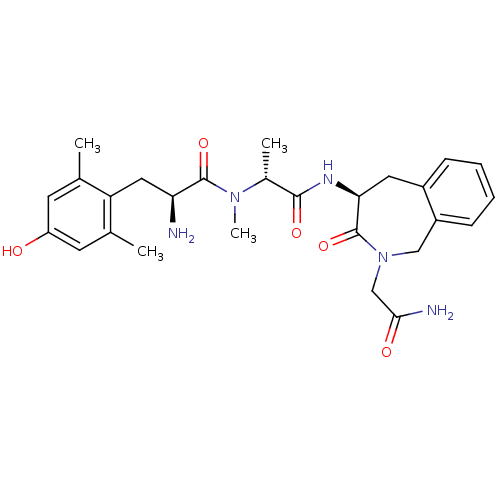
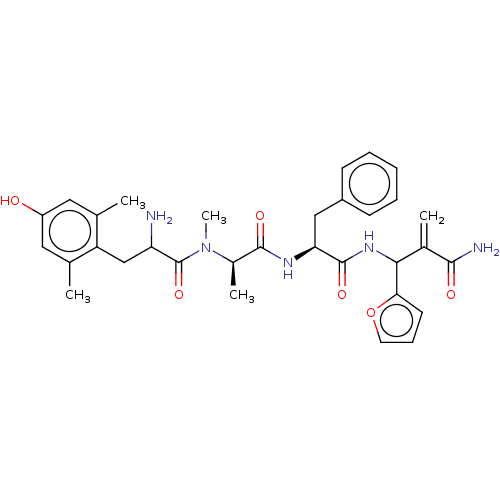
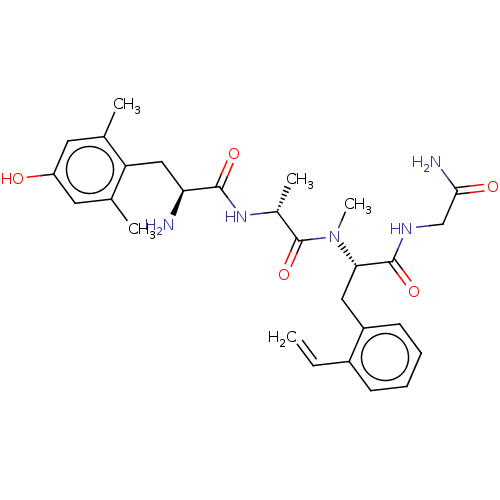
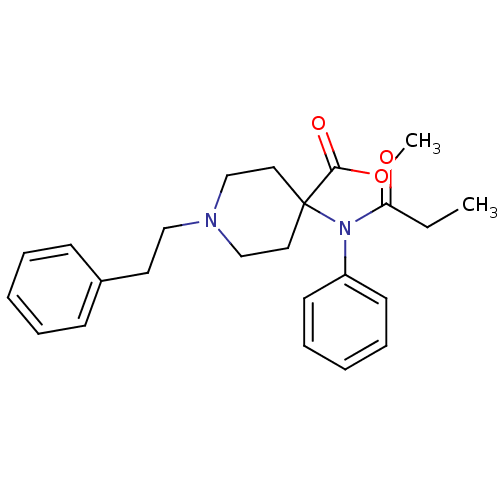
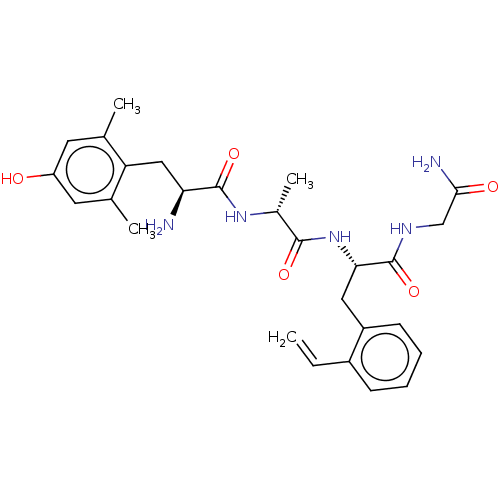
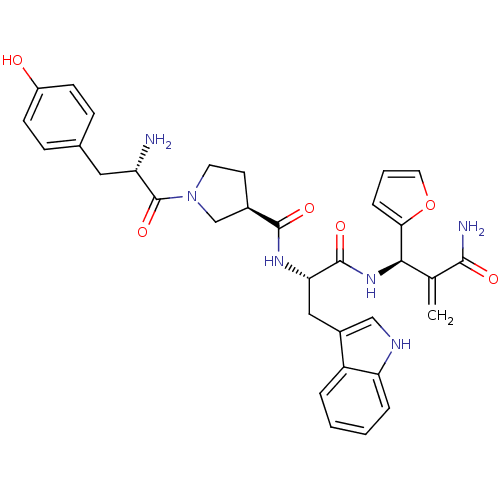
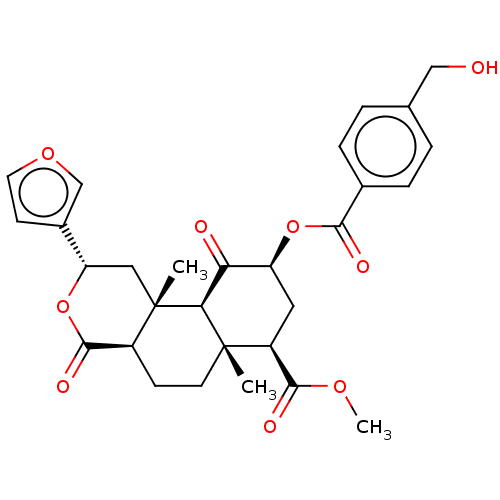
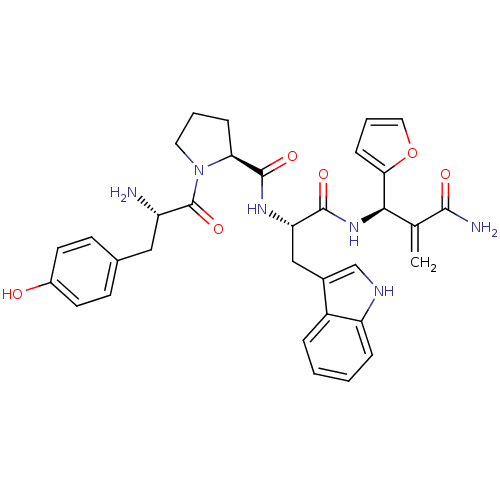
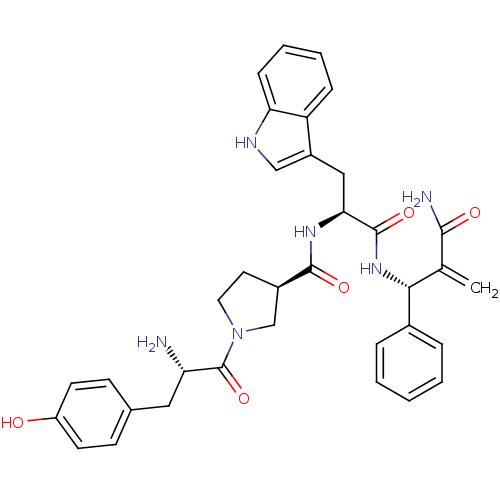
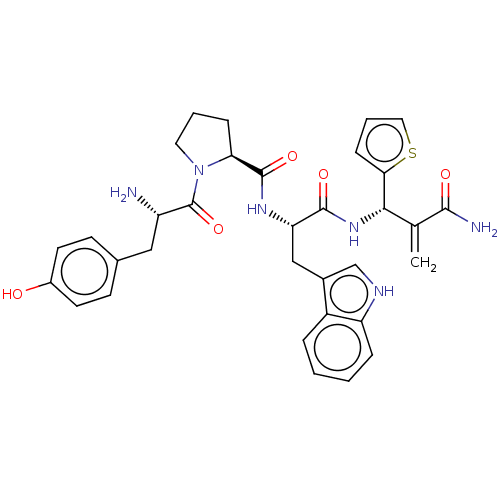
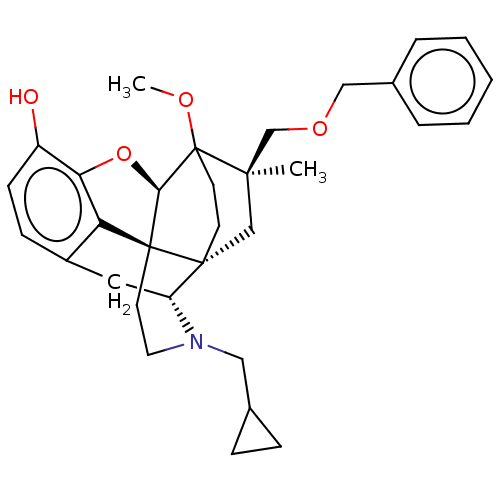
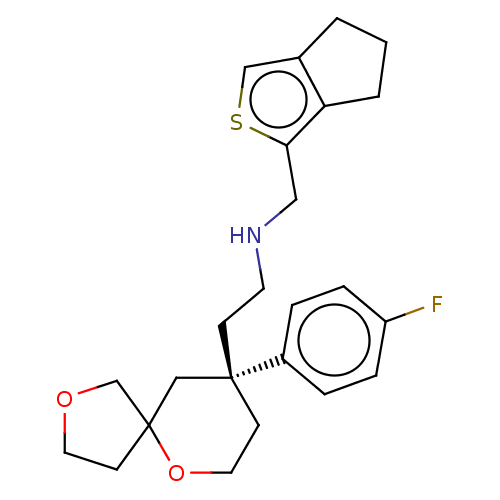
Affinity DataEC50: 0.0000420nMAssay Description:Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c...More data for this Ligand-Target Pair
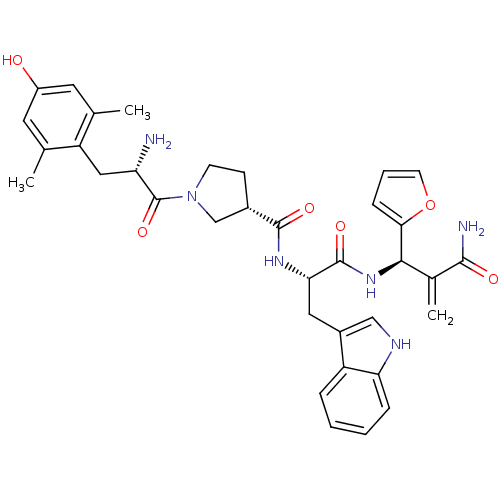
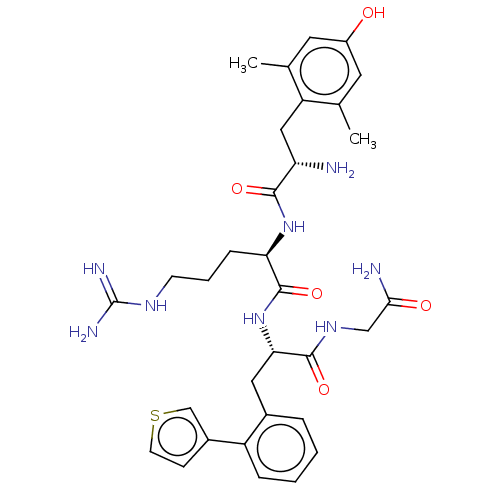
Affinity DataEC50: 0.0000910nMAssay Description:Agonist activity at FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAM...More data for this Ligand-Target Pair
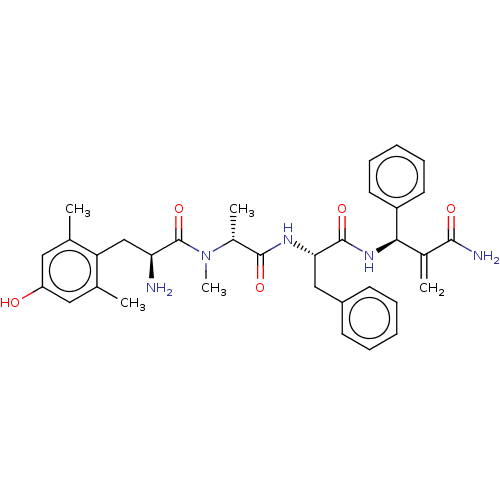
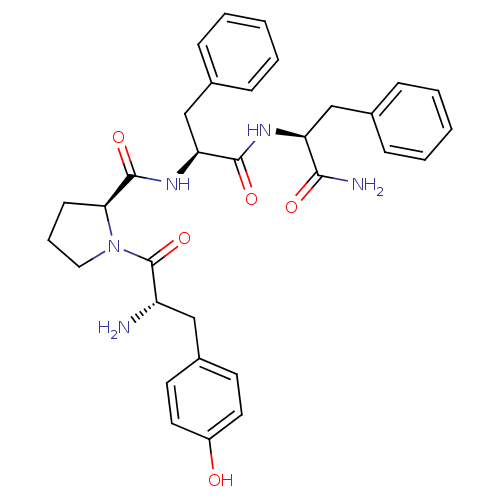
Affinity DataEC50: 0.000107nMAssay Description:Agonist activity at FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAM...More data for this Ligand-Target Pair
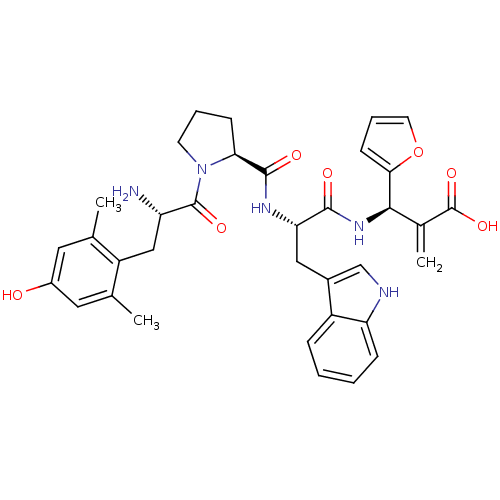
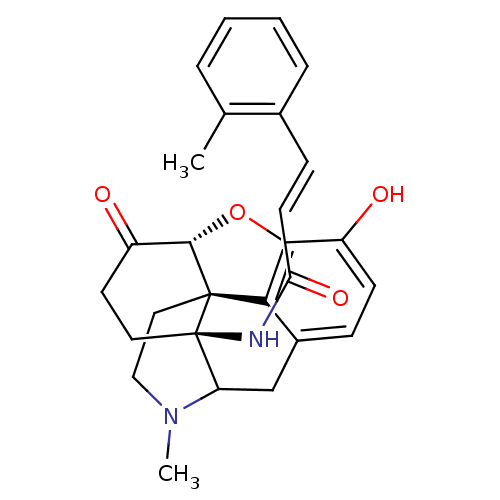
Affinity DataEC50: 0.000109nMAssay Description:Agonist activity at FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAM...More data for this Ligand-Target Pair
Affinity DataEC50: 0.000130nMAssay Description:Agonist activity at FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAM...More data for this Ligand-Target Pair
Affinity DataEC50: 0.000515nMAssay Description:Agonist activity at MOP (unknown origin) stably expressed in HEK293 cells assessed as forskolin stimulated cAMP accumulation incubated for 30 mins by...More data for this Ligand-Target Pair
Affinity DataEC50: 0.000516nMAssay Description:Agonist activity at FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAM...More data for this Ligand-Target Pair
Affinity DataEC50: 0.00100nMAssay Description:Activity at mu opioid receptor assessed as increase in calcium level in CHO cells by aequorin luminescence based calcium assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.00174nMAssay Description:Agonist activity at human MOR expressed in HEK293 cell membrane assessed as inhibition of forskolin-induced cAMP accumulation by by liquid scintillat...More data for this Ligand-Target Pair
Affinity DataEC50: 0.00177nMAssay Description:Displacement of [3H]DAMGO from mu opioid receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 0.00177nMAssay Description:Displacement of [3H]DAMGO from mu opioid receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 0.00177nMAssay Description:Displacement of [3H]DAMGO from mu opioid receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 0.00177nMAssay Description:Displacement of [3H]DAMGO from mu opioid receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 0.00180nMAssay Description:Agonist activity at MOP (unknown origin) stably expressed in HEK293 cells assessed as forskolin stimulated cAMP accumulation incubated for 30 mins by...More data for this Ligand-Target Pair
Affinity DataEC50: 0.00201nMAssay Description:Agonist activity at MOP (unknown origin) stably expressed in HEK293 cells assessed as forskolin stimulated cAMP accumulation incubated for 30 mins by...More data for this Ligand-Target Pair
Affinity DataEC50: 0.00240nMAssay Description:Agonist activity at human MOR expressed in CHO-K1 cells assessed as cAMP accumulation incubated for 30 mins and measured after 1 hr by Eu-cAMP tracer...More data for this Ligand-Target Pair
Affinity DataEC50: 0.00300nMAssay Description:Tested for thromboxane antagonist potency (pA2) against U 44619 induced platelet aggregation using human platelet rich plasmaMore data for this Ligand-Target Pair
Affinity DataEC50: 0.00400nMAssay Description:Agonist activity at MOP (unknown origin) stably expressed in HEK293 cells assessed as forskolin stimulated cAMP accumulation incubated for 30 mins by...More data for this Ligand-Target Pair
Affinity DataEC50: 0.00490nMAssay Description:Agonist activity at human MOR expressed in CHO-K1 cells assessed as cAMP accumulation incubated for 30 mins and measured after 1 hr by Eu-cAMP tracer...More data for this Ligand-Target Pair
Affinity DataEC50: 0.00510nMAssay Description:Agonist activity at human MOR expressed in CHO-K1 cells assessed as cAMP accumulation incubated for 30 mins and measured after 1 hr by Eu-cAMP tracer...More data for this Ligand-Target Pair
Affinity DataEC50: 0.00600nMAssay Description:Agonist activity at rat mu-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-ind...More data for this Ligand-Target Pair
Affinity DataEC50: 0.00800nMAssay Description:Agonist activity at rat mu-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-ind...More data for this Ligand-Target Pair
Affinity DataEC50: 0.00853nMAssay Description:Agonist activity at FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAM...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0120nMAssay Description:Agonist activity at rat mu-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-ind...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0120nMAssay Description:Agonist activity at rat mu-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-ind...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0140nMAssay Description:Agonist activity at human MOR expressed in CHO-K1 cells assessed as cAMP accumulation incubated for 30 mins and measured after 1 hr by Eu-cAMP tracer...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0170nMAssay Description:Agonist activity at human MOR expressed in CHOK1 cells assessed as inhibition of forskolin-stimulated cAMP accumulation incubated for 30 mins by lumi...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0280nMAssay Description:Agonist activity at human MOR expressed in CHO-K1 cells assessed as cAMP accumulation incubated for 30 mins and measured after 1 hr by Eu-cAMP tracer...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0300nMAssay Description:Agonist activity at human MOP stably expressed in CHO-K1 cells assessed as inhibition of forskolin-induced cAMP accumulationMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0334nMAssay Description:Agonist activity at mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins by liqui...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0342nMAssay Description:Agonist activity at mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins by liqui...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0397nMAssay Description:Agonist activity at FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAM...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0400nMAssay Description:The experiment was performed using a cAMP detection kit from Cisbio (Cisbio #62AM4PEJ).More data for this Ligand-Target Pair
Affinity DataEC50: 0.0400nMAssay Description:Agonist activity at rat mu-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-ind...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0400nMAssay Description:Stimulation of [35S]GTPgammaS binding to human recombinant MORMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0400nMAssay Description:Antagonist activity at mu opioid receptor expressed in CHO cells assessed as release of intracellular calcium ions by aequorin luminescence-based cal...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0400nMAssay Description:Stimulation of [35S]GTPgammaS binding to human recombinant MORMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0400nMAssay Description:Activity at mu opioid receptor assessed as increase in calcium level in CHO cells by aequorin luminescence based calcium assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0570nMAssay Description:Agonist activity at FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAM...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0600nMAssay Description:Agonist activity at rat mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0600nMAssay Description:Agonist activity at rat mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0629nMAssay Description:Agonist activity at mu opioid receptor (unknown origin) transfected in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP productionMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0640nMAssay Description:μ-Opioid:Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.),...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0643nMAssay Description:Agonist activity at mu opioid receptor (unknown origin) transfected in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP productionMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0700nMAssay Description:The experiment was performed using a cAMP detection kit from Cisbio (Cisbio #62AM4PEJ).More data for this Ligand-Target Pair
Affinity DataEC50: 0.0700nMAssay Description:Agonist activity at mu opioid receptor (unknown origin) assessed as increase in cAMP level incubated for 40 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0750nMAssay Description:The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0750nMAssay Description:The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0790nMAssay Description:Agonist activity at human MOR expressed in HEK293 cell membrane assessed as inhibition of forskolin-induced cAMP accumulation by by liquid scintillat...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0832nMAssay Description:Agonist activity at human MOR expressed in HEK293 cell membrane assessed as inhibition of forskolin-induced cAMP accumulation by by liquid scintillat...More data for this Ligand-Target Pair