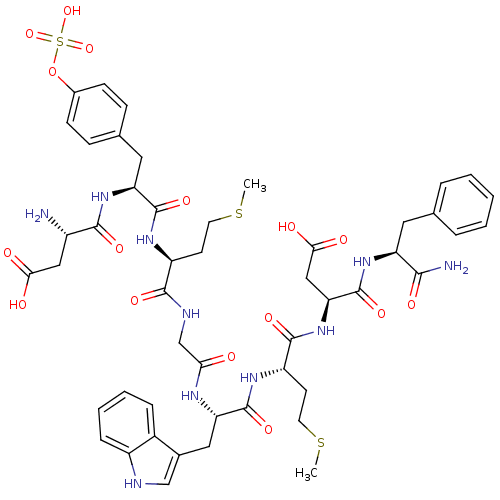
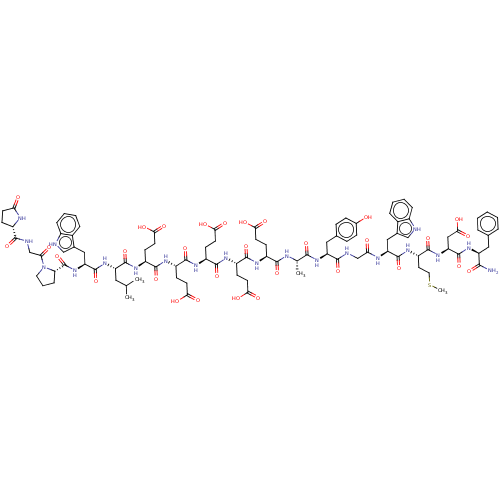
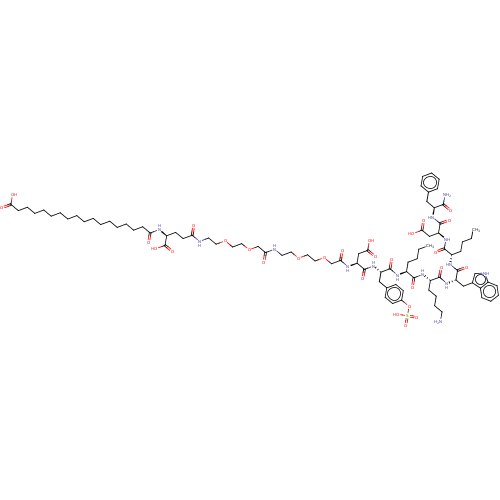
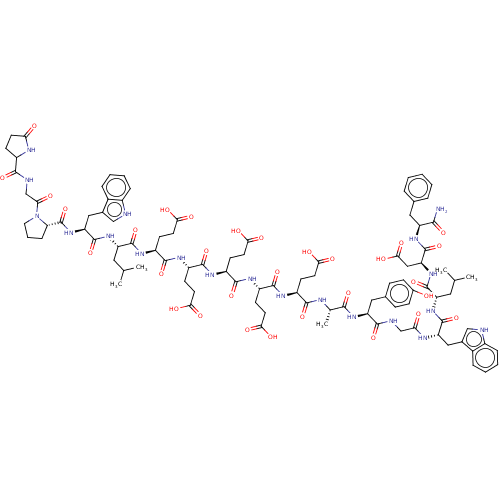
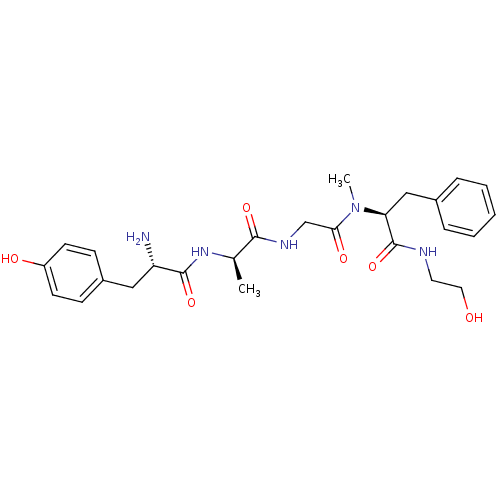
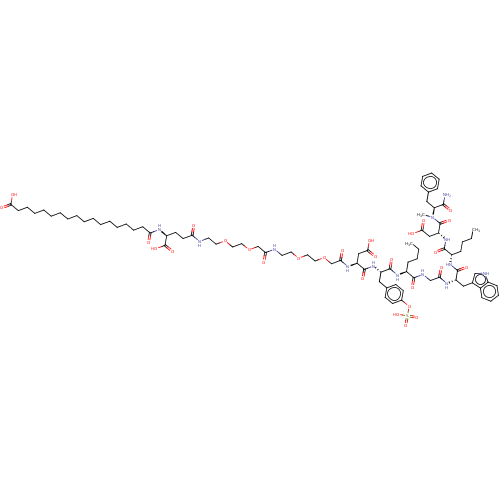
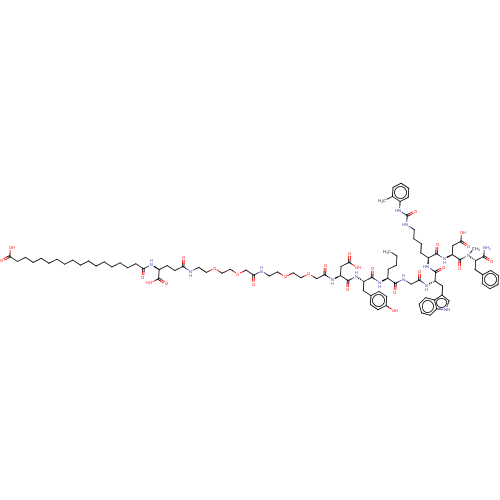
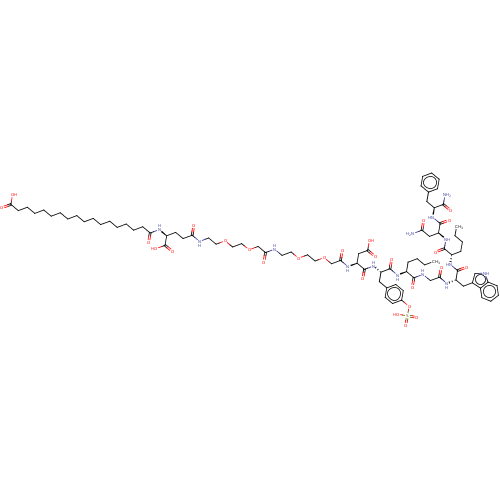
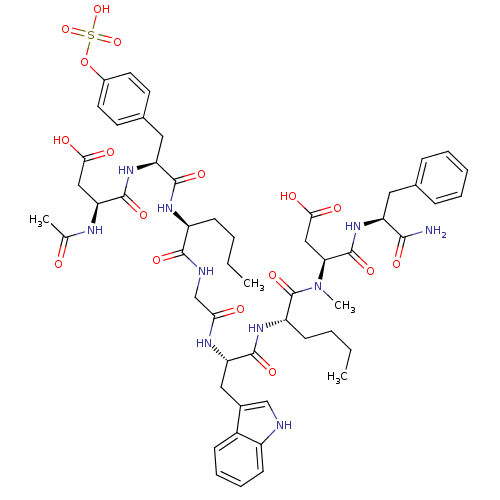
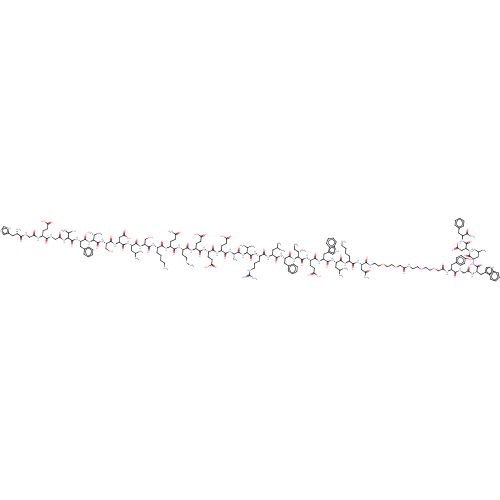
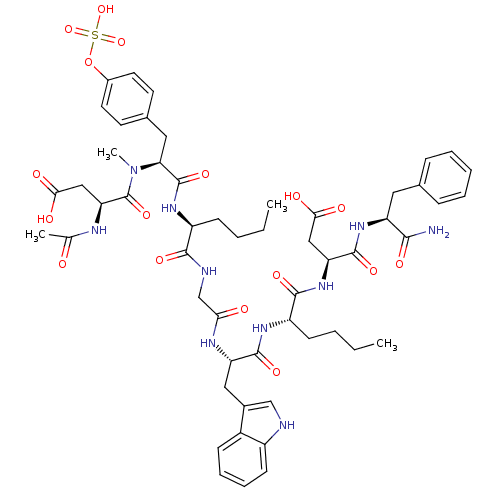
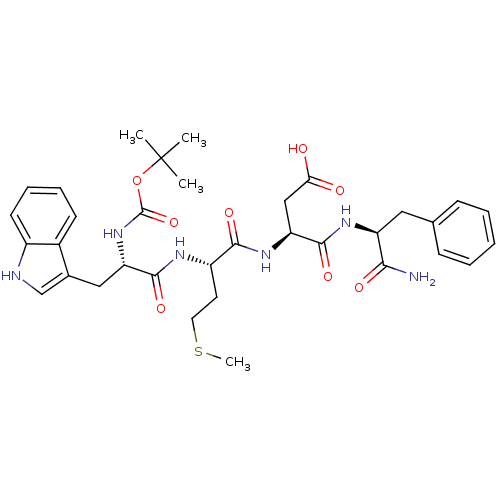
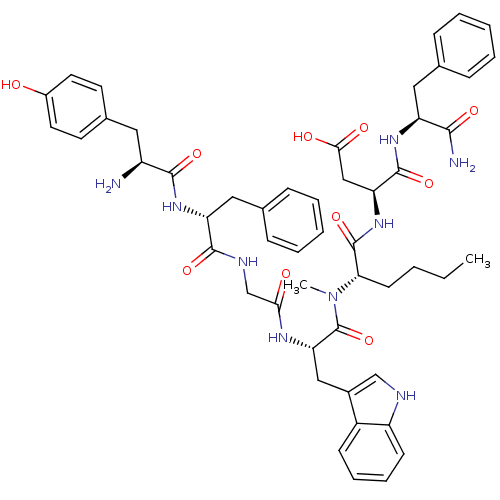
Affinity DataEC50: 0.0930nMAssay Description:Displacement of [125I]CCK-8 from CCK2 receptor in human FGS7 Jurkat cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.295nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.316nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.324nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.331nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.380nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.380nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.407nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.417nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.417nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.437nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.437nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.447nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.457nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.457nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.501nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.501nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.631nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.832nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1.5nMAssay Description:Inhibition of (Thr,Nle)-CCK-9 -induced inositol phosphate production in COS-7 cells expressing human CCK2 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 1.5nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1.90nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2.40nMAssay Description:Agonist activity at human CCK2R expressed in HEK293 cells assessed as ERK1/2 phosphorylation incubated for 5 mins by AlphaScreen assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2.66nMAssay Description:Activity at CCK2R-MOPR (unknown origin) coexpressed in CHO cells cotransfected with delta6-Galphaqi4-myr assessed as intracellular calcium release by...More data for this Ligand-Target Pair
Affinity DataEC50: 2.70nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nM EC50: 2.80nMAssay Description:Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe...More data for this Ligand-Target Pair
Affinity DataEC50: 2.80nMAssay Description:Agonist activity at human CCK2R expressed in human A431 cells assessed as intracellular calcium mobilization measured after 24 hrs by Fluo-4AM dye ba...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nM EC50: 4.60nMAssay Description:Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe...More data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 5.80nMAssay Description:Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 6nMAssay Description:In vitro smooth muscle contraction activity in guinea pig stomach consisting of CCK/gastrin receptor subtypeMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nM EC50: 6.60nMAssay Description:Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe...More data for this Ligand-Target Pair
Affinity DataEC50: 7.30nMAssay Description:Agonist activity at human CCK-2R expressed in HEK293 cells assessed as stimulation of ERK phosphorylation incubated for 5 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 7.60nMAssay Description:Agonist activity at human CCK-2R expressed in HEK293 cells assessed as stimulation of ERK phosphorylation incubated for 5 minsMore data for this Ligand-Target Pair
Affinity DataKi: 150nM EC50: 8nMAssay Description:Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe...More data for this Ligand-Target Pair
Affinity DataEC50: 8.20nMAssay Description:Agonist activity at human CCK-2R expressed in HEK293 cells assessed as stimulation of ERK phosphorylation incubated for 5 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 8.30nMAssay Description:Agonist activity at human CCK-2R expressed in HEK293 cells assessed as stimulation of ERK phosphorylation incubated for 5 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 8.80nMAssay Description:Agonist activity at human CCK-2R expressed in HEK293 cells assessed as stimulation of ERK phosphorylation incubated for 5 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 9nMAssay Description:In vitro smooth muscle contraction activity in guinea pig stomach consisting of CCK/gastrin receptor subtypeMore data for this Ligand-Target Pair
Affinity DataEC50: 9.40nMAssay Description:Agonist activity at human CCK-2R expressed in HEK293 cells assessed as stimulation of ERK phosphorylation incubated for 5 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 9.90nMAssay Description:Agonist activity at human CCK-2R expressed in HEK293 cells assessed as stimulation of ERK phosphorylation incubated for 5 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 12nMAssay Description:In vitro smooth muscle contraction activity in guinea pig stomach consisting of CCK/gastrin receptor subtypeMore data for this Ligand-Target Pair
Affinity DataEC50: 12nMAssay Description:Agonist activity at human CCK2R expressed in human A431 cells assessed as intracellular calcium mobilization measured after 24 hrs by Fluo-4AM dye ba...More data for this Ligand-Target Pair
Affinity DataEC50: 12nMAssay Description:Agonist activity at human CCK2R expressed in human A431 cells assessed as intracellular calcium mobilization measured after 24 hrs by Fluo-4AM dye ba...More data for this Ligand-Target Pair
Affinity DataEC50: 13nMAssay Description:Agonist activity at CCKBR in human NCI-H345 cells assessed as calcium releaseMore data for this Ligand-Target Pair
Affinity DataEC50: 14nMAssay Description:Agonist activity at human CCK2R expressed in human A431 cells assessed as intracellular calcium mobilization measured after 24 hrs by Fluo-4AM dye ba...More data for this Ligand-Target Pair
Affinity DataEC50: 14nMAssay Description:In vitro smooth muscle contraction activity in guinea pig stomach consisting of CCK/gastrin receptor subtypeMore data for this Ligand-Target Pair
Affinity DataEC50: 15nMAssay Description:Agonist activity at human CCK-2R expressed in HEK293 cells assessed as stimulation of ERK phosphorylation incubated for 5 minsMore data for this Ligand-Target Pair
Affinity DataKi: 15nM EC50: 16nMAssay Description:Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe...More data for this Ligand-Target Pair
Affinity DataEC50: 16nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair