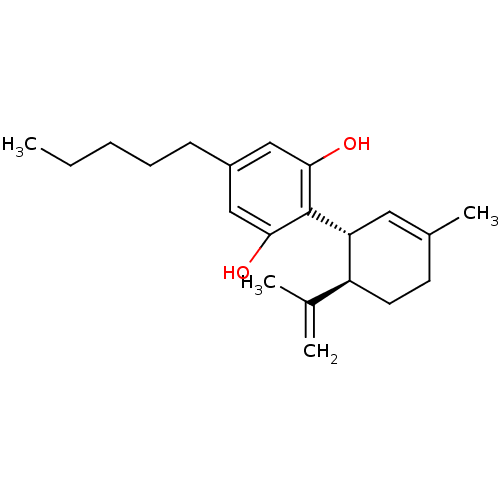
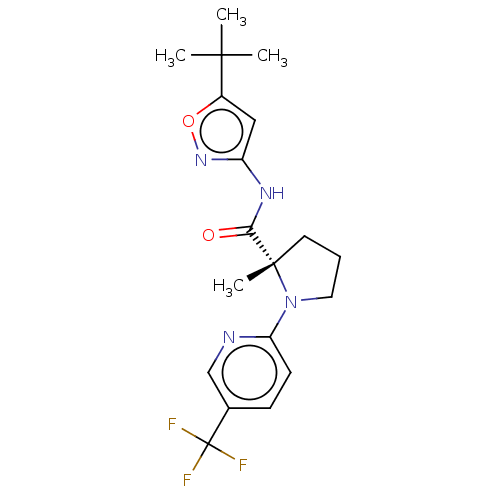
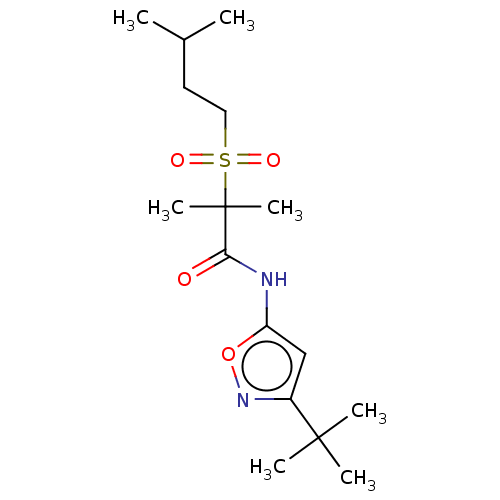
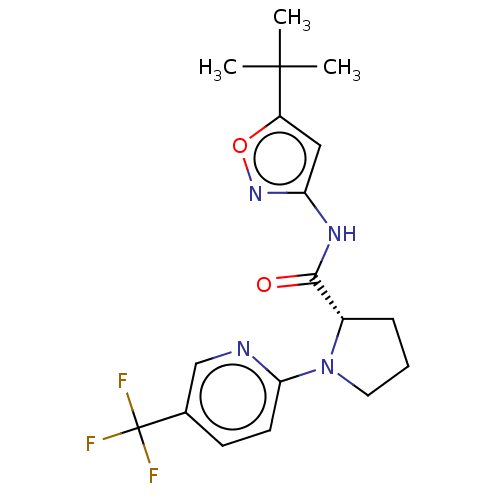
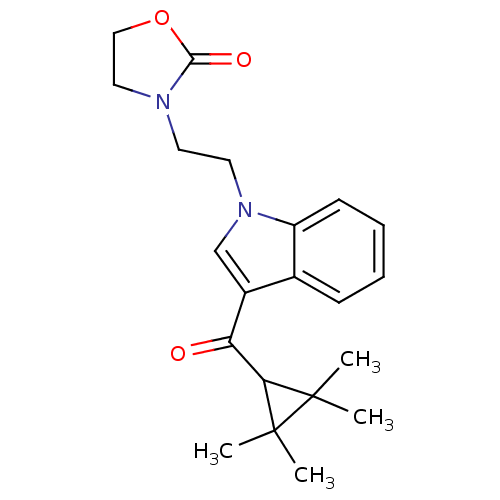
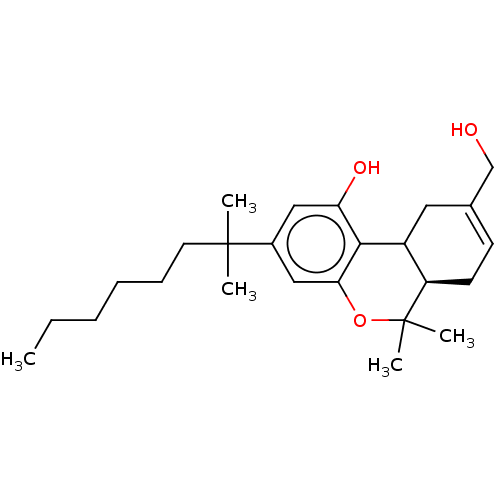
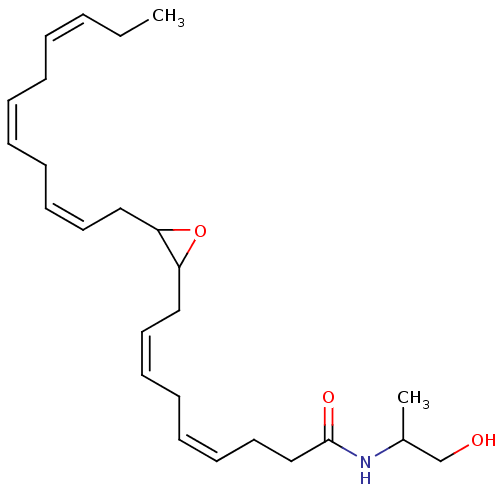
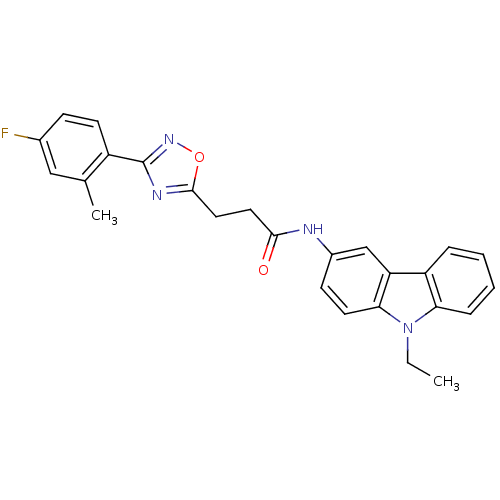
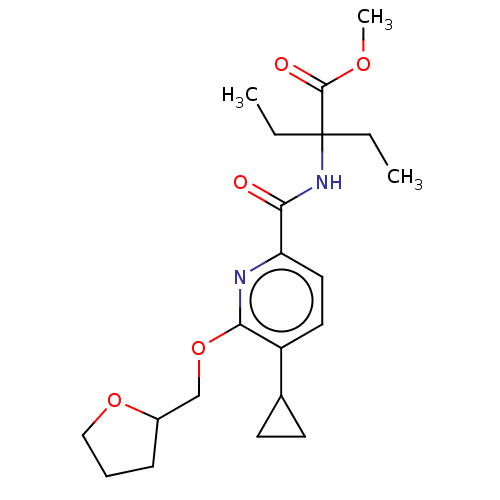
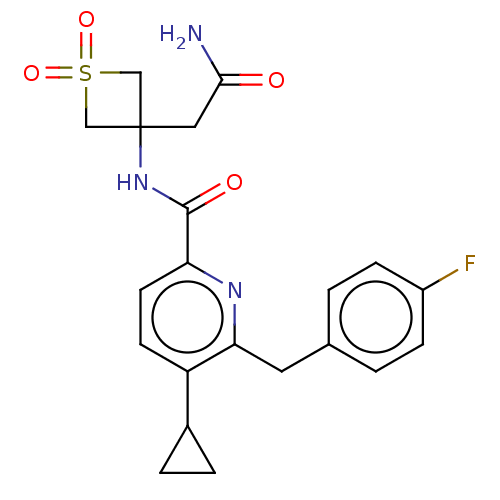
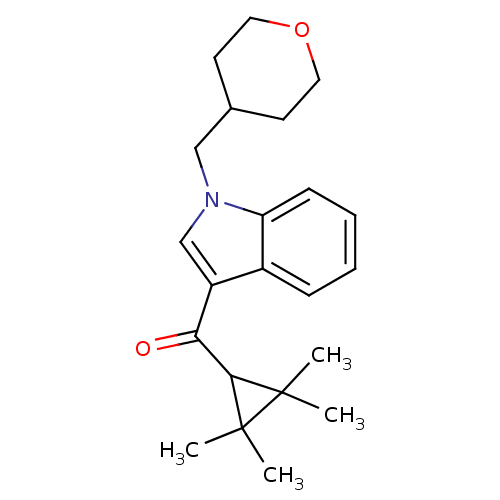
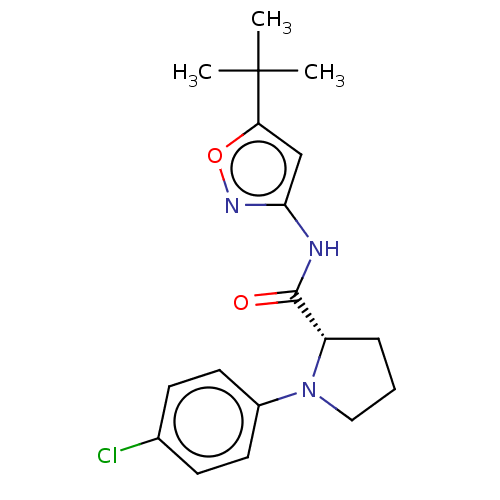
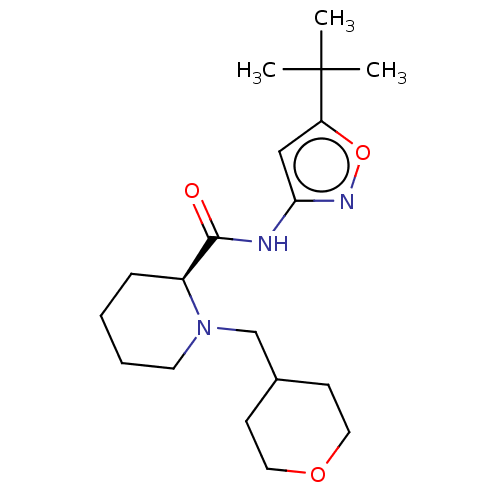
Affinity DataEC50: 0nMAssay Description:Partial agonist activity at CBR2 A2827.36M mutant (unknown origin) expressed in HEK293T assessed as increase in picometer shifts of reflected light w...More data for this Ligand-Target Pair
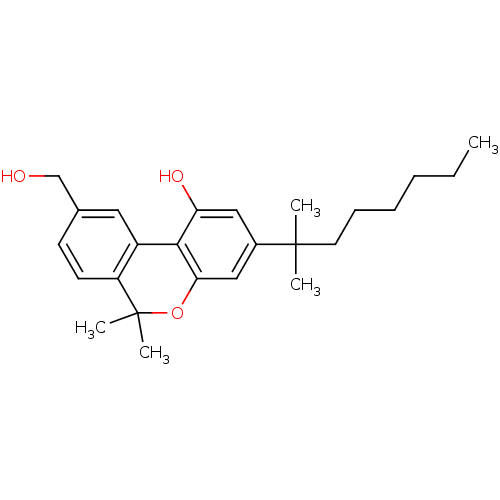
Affinity DataEC50: 0nMAssay Description:Partial agonist activity at CBR2 A2827.36M mutant (unknown origin) expressed in HEK293T assessed as increase in picometer shifts of reflected light w...More data for this Ligand-Target Pair
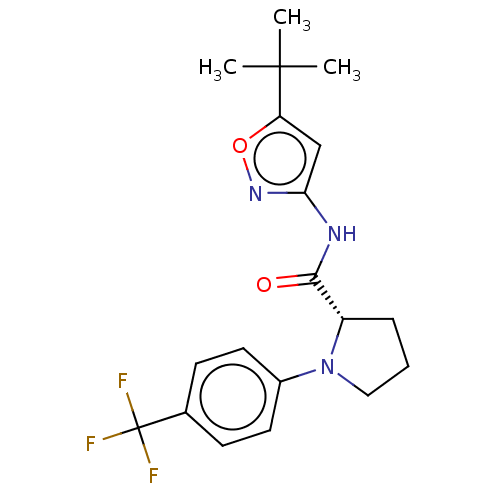
Affinity DataEC50: 0nMAssay Description:Partial agonist activity at CBR2 A2827.36M mutant (unknown origin) expressed in HEK293T assessed as increase in picometer shifts of reflected light w...More data for this Ligand-Target Pair
Affinity DataEC50: 0nMAssay Description:Negative allosteric modulation of JWH-133-induced agonist activity at wild type CB2 receptor (unknown origin) stably expressed in HEK293T cells asses...More data for this Ligand-Target Pair
Affinity DataEC50: 0nMAssay Description:Partial agonist activity at CBR2 S2857.39L mutant (unknown origin) expressed in HEK293T assessed as increase in picometer shifts of reflected light w...More data for this Ligand-Target Pair
Affinity DataEC50: 0nMAssay Description:Partial agonist activity at CBR2 S2857.39L mutant (unknown origin) expressed in HEK293T assessed as increase in picometer shifts of reflected light w...More data for this Ligand-Target Pair
Affinity DataEC50: 0nMAssay Description:Partial agonist activity at CBR2 S2857.39L mutant (unknown origin) expressed in HEK293T assessed as increase in picometer shifts of reflected light w...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0150nMAssay Description:Agonist activity at human recombinant CB2 receptor expressed in Sf9 cells by GTP-europium binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0400nMAssay Description:Inhibition of human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0400nMAssay Description:Agonist activity at human CB2 receptor assessed as inhibition of forskolin-induced cAMP production preincubated for 15 mins followed by forskolin cha...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0670nMAssay Description:Displacement of [3H]-CP55940 from human CB2 receptor expressed in HEK293 cells after 90 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0700nMAssay Description:Agonist activity at human recombinant CB2 receptor expressing CHO cells assessed as inhibition of forskolin-induced cAMP accumulation incubated for 3...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0700nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0700nMAssay Description:Agonist activity at rat CB2 receptor assessed as inhibition of forskolin-induced cAMP production by cell based assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0700nMAssay Description:Inhibition of human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0780nMAssay Description:Effective concentration for inhibition of human Cannabinoid receptor 2-mediated adenylylcyclase using African green monkey (COS-7) cells transfected ...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0800nMAssay Description:Inhibition of human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0800nMAssay Description:Binding of the parent compounds to putative receptors CNR1 and CNR2 (cannabinoid receptors 1& 2) was measured by a Presto-Tango assay (FIG. 5A and FI...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0800nMAssay Description:Agonist activity at N-terminal FLAG-tagged human CB2 receptor transfected in human HTLA cells assessed as induction of beta-arrestin-recruitment afte...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0890nMAssay Description:Agonist activity at human recombinant CB2 receptor expressed in Sf9 cells by GTP-europium binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0900nMAssay Description:Inhibition of human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0930nMAssay Description:Agonist activity at human recombinant CB2 receptor expressing CHO cells assessed as inhibition of forskolin-induced cAMP accumulation incubated for 3...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0930nMAssay Description:Displacement of [3H]-CP55940 from human CB2 receptor expressed in HEK293 cells after 90 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0930nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.100nMT: 2°CAssay Description:CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl...More data for this Ligand-Target Pair
Affinity DataEC50: 0.100nMT: 2°CAssay Description:CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl...More data for this Ligand-Target Pair
Affinity DataEC50: 0.130nMAssay Description:Agonist activity at rat recombinant CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated intracellular cAMP levelMore data for this Ligand-Target Pair
Affinity DataEC50: 0.150nMAssay Description:Agonist activity at human CB2 receptor transfected in CHO-K1 cells assessed as cAMP changeMore data for this Ligand-Target Pair
Affinity DataEC50: 0.170nMAssay Description:Agonist activity at rat CB2 receptor assessed as inhibition of forskolin-induced cAMP production by cell based assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.190nMAssay Description:Inhibition of human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.190nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.190nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at human recombinant CB2 receptor expressed in Sf9 cells by GTP-europium binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at human recombinant CB2 receptor expressing CHO cells assessed as inhibition of forskolin-induced cAMP accumulation incubated for 3...More data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at human recombinant CB2 receptor expressing CHO cells assessed as inhibition of forskolin-induced cAMP accumulation incubated for 3...More data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF methodMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMT: 2°CAssay Description:CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl...More data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMT: 2°CAssay Description:CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl...More data for this Ligand-Target Pair
Affinity DataEC50: 0.208nMAssay Description:Effective concentration for inhibition of human Cannabinoid receptor 2-mediated adenylylcyclase using African green monkey (COS-7) cells transfected ...More data for this Ligand-Target Pair
Affinity DataEC50: 0.210nMAssay Description:Inhibition of human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.230nMAssay Description:CHO cells expressing human CB2R (Euroscreen) were plated at a density of 10,000 cells per well in 384 well plates and incubated overnight at 37° C. A...More data for this Ligand-Target Pair
Affinity DataEC50: 0.230nMAssay Description:CHO cells expressing human CB2R (Euroscreen) were plated at a density of 10,000 cells per well in 384 well plates and incubated overnight at 37° C. A...More data for this Ligand-Target Pair
Affinity DataEC50: 0.230nMAssay Description:Inhibition of human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.230nMAssay Description:CB2: CHO cells expressing human CB2R (Euroscreen) were plated at a density of 10,000 cells per well in 384 well plates and incubated overnight at 37°...More data for this Ligand-Target Pair
Affinity DataEC50: 0.230nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.230nMAssay Description:CHO cells expressing human CB2R (Euroscreen) were plated at a density of 10,000 cells per well in 384 well plates and incubated overnight at 37° C. A...More data for this Ligand-Target Pair
Affinity DataEC50: 0.25nMT: 2°CAssay Description:CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl...More data for this Ligand-Target Pair
Affinity DataEC50: 0.25nMT: 2°CAssay Description:CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl...More data for this Ligand-Target Pair
Affinity DataEC50: 0.251nMAssay Description:Agonist activity at human CB2 receptor transfected in CHO cells by [35]GTPgamma binding assayMore data for this Ligand-Target Pair