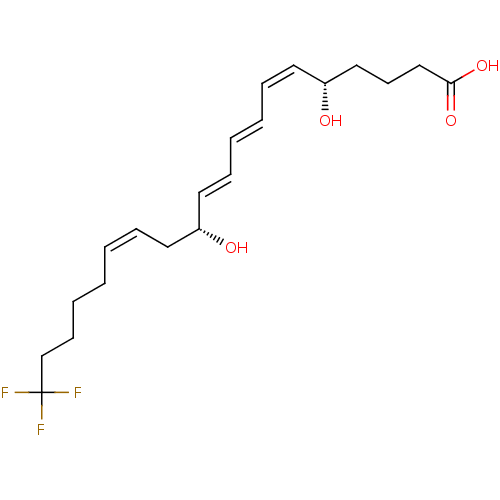
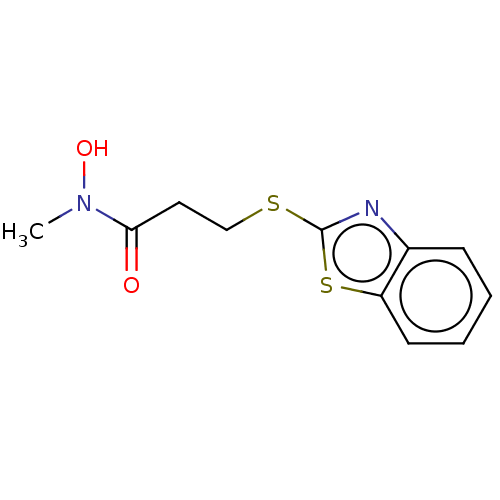
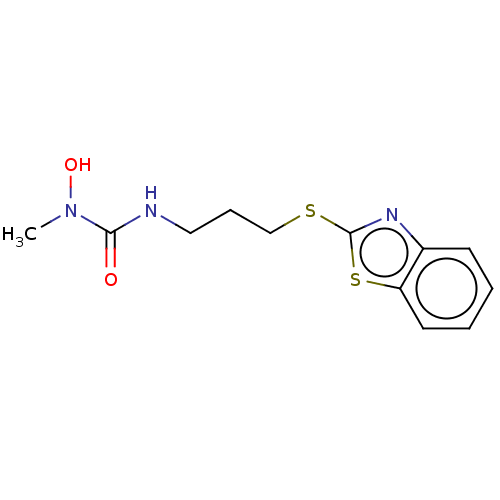
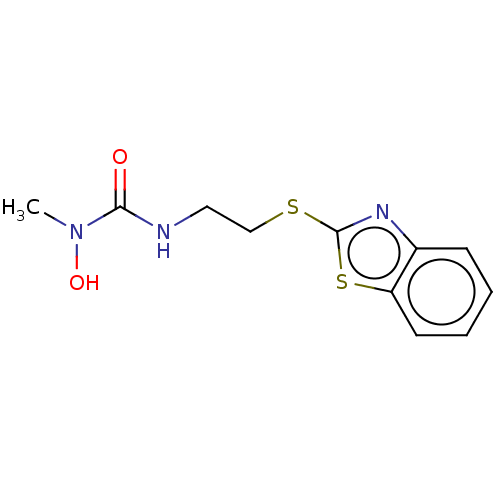
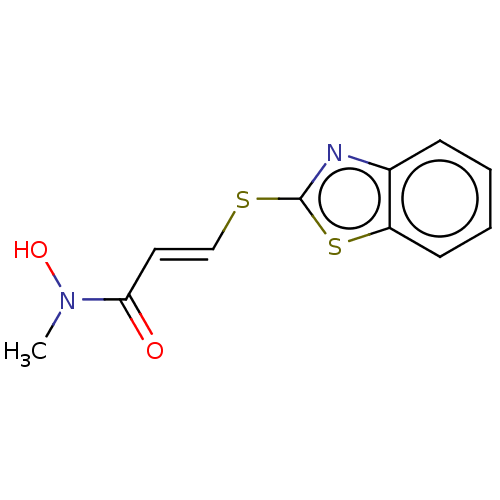
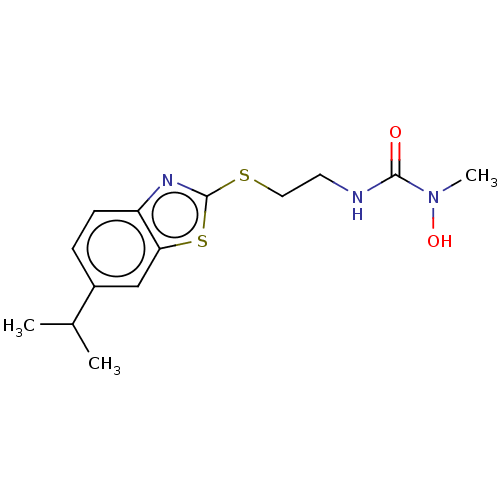
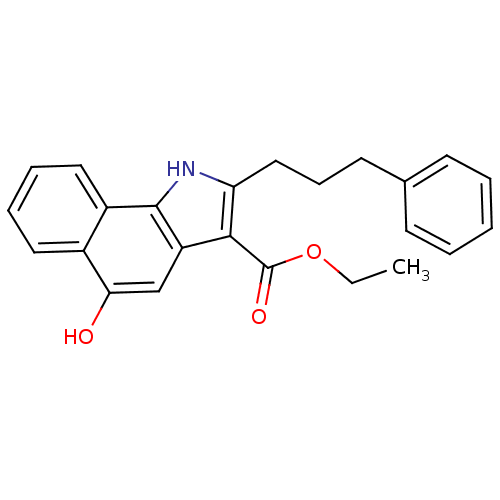
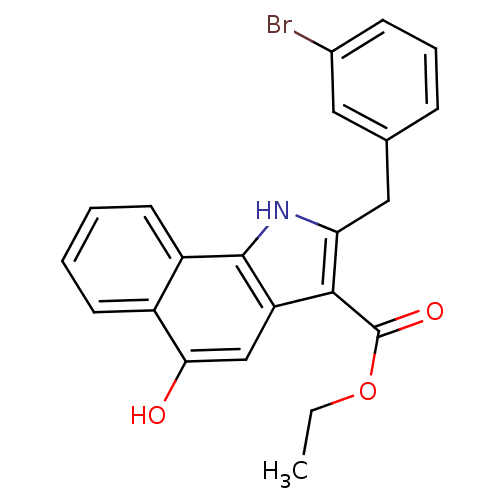
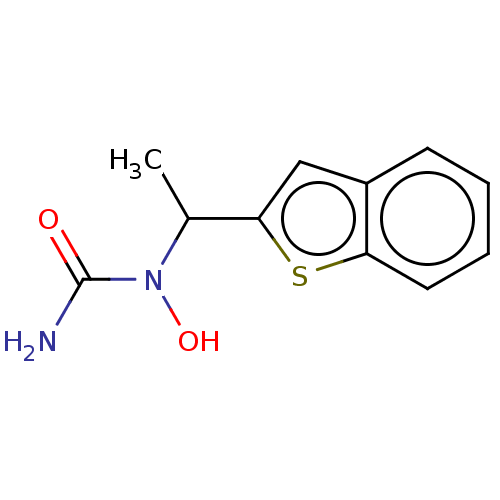
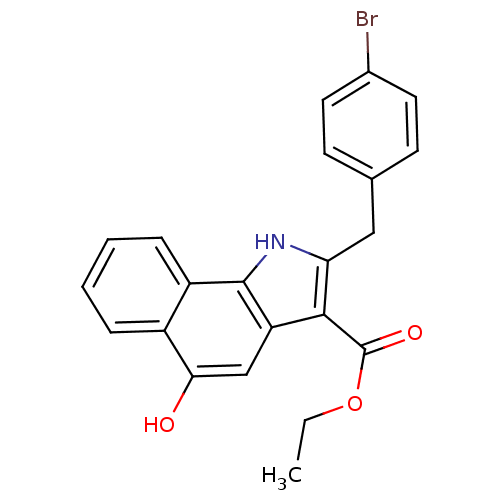
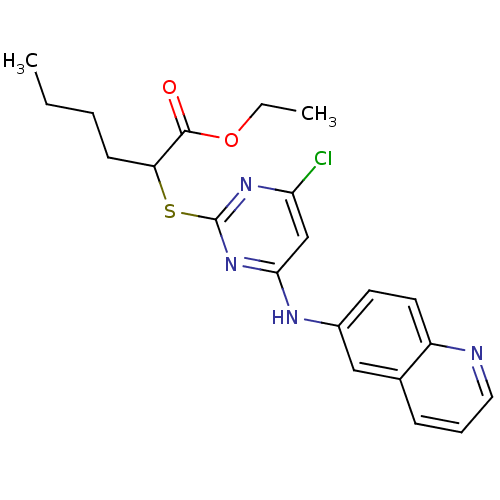
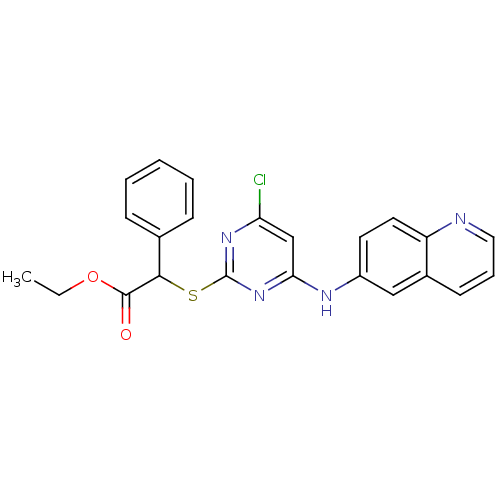
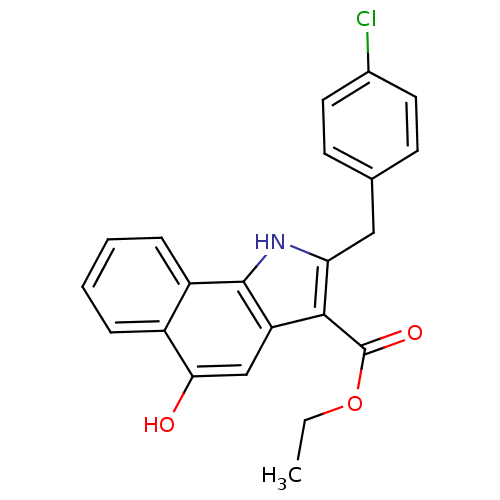
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 3nMAssay Description:Effective concentration required to inhibit 5-lipoxygenase by 50%More data for this Ligand-Target Pair
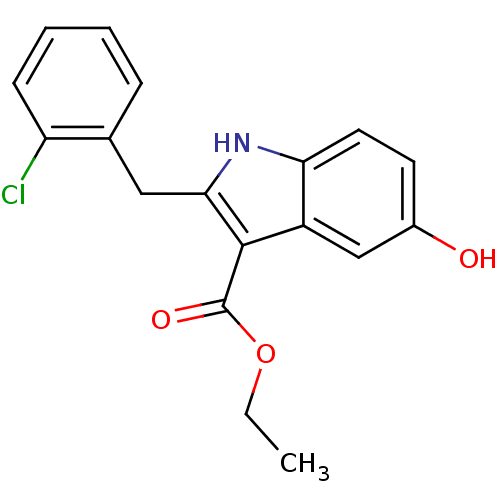
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 3nMAssay Description:Effective concentration required to inhibit 5-lipoxygenase by 50%More data for this Ligand-Target Pair
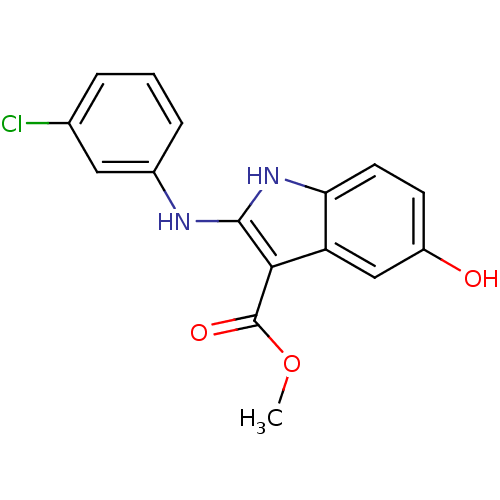
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 20nMAssay Description:In vitro inhibition of ionophore stimulated LTB4 release from human peripheral blood leukocytes.More data for this Ligand-Target Pair
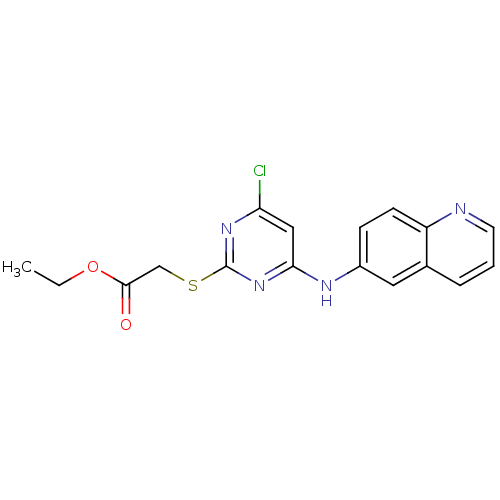
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 20nMAssay Description:In vitro inhibition of ionophore stimulated LTB4 release from human peripheral blood leukocytes.More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 39nMAssay Description:In vitro inhibition of ionophore stimulated LTB4 release from human peripheral blood leukocytes.More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 45nMAssay Description:In vitro inhibition of ionophore stimulated LTB4 release from human peripheral blood leukocytes.More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 70nMAssay Description:In vitro inhibition of ionophore stimulated LTB4 release from human peripheral blood leukocytes.More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 70nMAssay Description:In vitro inhibition of ionophore stimulated LTB4 release from human peripheral blood leukocytes.More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 70nMAssay Description:In vitro inhibition of ionophore stimulated LTB4 release from human peripheral blood leukocytes.More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 70nMAssay Description:In vitro inhibition of ionophore stimulated LTB4 release from human peripheral blood leukocytes.More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 90nMAssay Description:In vitro inhibition of ionophore stimulated LTB4 release from human peripheral blood leukocytes.More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 110nMAssay Description:In vitro inhibition of ionophore stimulated LTB4 release from human peripheral blood leukocytes.More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 130nMAssay Description:In vitro inhibition of ionophore stimulated LTB4 release from human peripheral blood leukocytes.More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 130nMAssay Description:In vitro inhibition of ionophore stimulated LTB4 release from human peripheral blood leukocytes.More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 160nMAssay Description:In vitro inhibition of ionophore stimulated LTB4 release from human peripheral blood leukocytes.More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 86nM EC50: 230nMpH: 7.4 T: 2°CAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 67nM EC50: 320nMAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 140nM EC50: 340nMpH: 7.4 T: 2°CAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 130nM EC50: 350nMAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 390nMAssay Description:In vitro inhibition of ionophore stimulated LTB4 release from human peripheral blood leukocytes.More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 130nM EC50: 440nMAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 150nM EC50: 450nMpH: 7.4 T: 2°CAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 470nMAssay Description:In vitro inhibition of ionophore stimulated LTB4 release from human peripheral blood leukocytes.More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 170nM EC50: 480nMAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 31nM EC50: 490nMAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 96nM EC50: 500nMpH: 7.4 T: 2°CAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 130nM EC50: 520nMpH: 7.4 T: 2°CAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 45nM EC50: 520nMpH: 7.4 T: 2°CAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 530nMAssay Description:In vitro inhibition of ionophore stimulated LTB4 release from human peripheral blood leukocytes.More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 95nM EC50: 600nMpH: 7.4 T: 2°CAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.90E+4nM EC50: 600nMpH: 7.4 T: 2°CAssay Description:For determination of the activity of 5-LO in 100000g supernatants, aliquots of the supernatants were added to reaction mix, and were preincubated wit...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 150nM EC50: 650nMpH: 7.4 T: 2°CAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nM EC50: 700nMpH: 7.4 T: 2°CAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 240nM EC50: 710nMpH: 7.4 T: 2°CAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 1.07E+3nMAssay Description:In vitro inhibition of ionophore stimulated LTB4 release from human peripheral blood leukocytes.More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 1.10E+3nMpH: 7.4 T: 2°CAssay Description:For assays of intact cells, 5000,000 freshly isolated PMNL cells were resuspended in PGC buffer. After preincubation with the test compounds for 15 m...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 1.20E+3nMpH: 7.4 T: 2°CAssay Description:For assays of intact cells, 5000,000 freshly isolated PMNL cells were resuspended in PGC buffer. After preincubation with the test compounds for 15 m...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 84nM EC50: 1.20E+3nMpH: 7.4 T: 2°CAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 97nM EC50: 1.20E+3nMpH: 7.4 T: 2°CAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.70E+4nM EC50: 1.40E+3nMpH: 7.4 T: 2°CAssay Description:For determination of the activity of 5-LO in 100000g supernatants, aliquots of the supernatants were added to reaction mix, and were preincubated wit...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 1.70E+3nMpH: 7.4 T: 2°CAssay Description:For assays of intact cells, 5000,000 freshly isolated PMNL cells were resuspended in PGC buffer. After preincubation with the test compounds for 15 m...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nM EC50: 1.70E+3nMpH: 7.4 T: 2°CAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 250nM EC50: 1.70E+3nMpH: 7.4 T: 2°CAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nM EC50: 1.70E+3nMpH: 7.4 T: 2°CAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 650nM EC50: 1.80E+3nMAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 1.80E+3nMpH: 7.4 T: 2°CAssay Description:For assays of intact cells, 5000,000 freshly isolated PMNL cells were resuspended in PGC buffer. After preincubation with the test compounds for 15 m...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nM EC50: 2.00E+3nMpH: 7.4 T: 2°CAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 300nM EC50: 2.40E+3nMpH: 7.4 T: 2°CAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 2.60E+3nMpH: 7.4 T: 2°CAssay Description:For assays of intact cells, 5000,000 freshly isolated PMNL cells were resuspended in PGC buffer. After preincubation with the test compounds for 15 m...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 49nM EC50: 2.80E+3nMAssay Description:For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated...More data for this Ligand-Target Pair