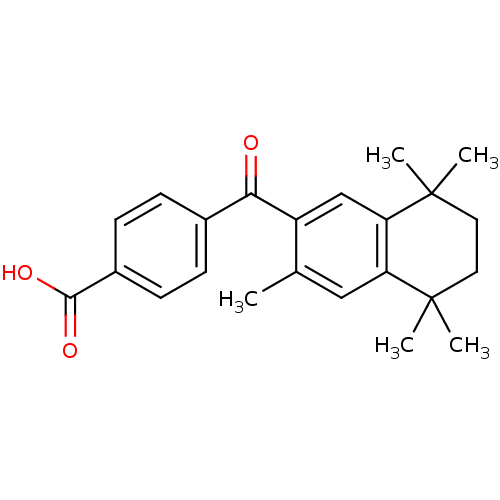
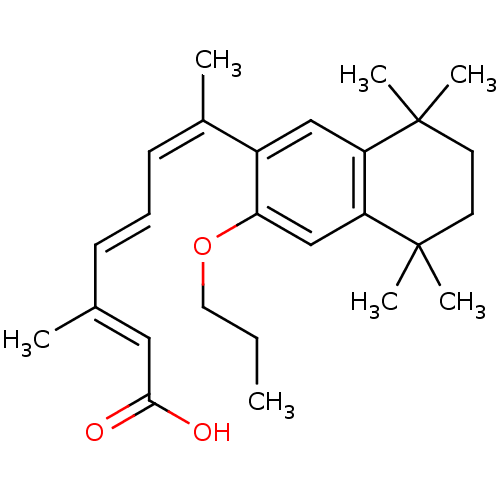
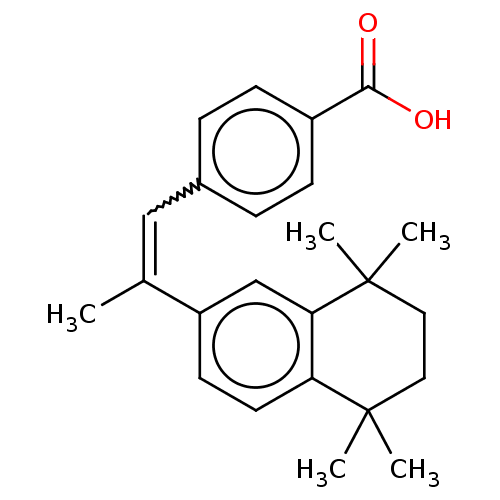
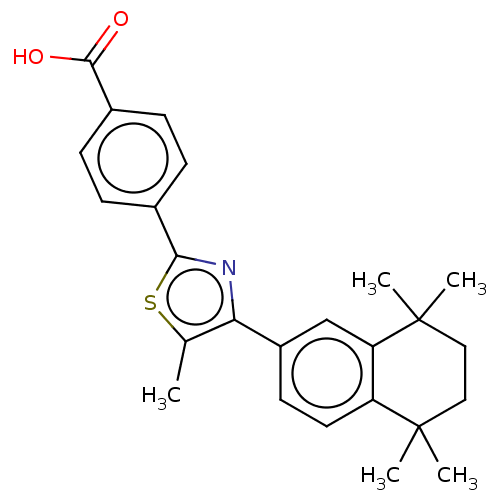
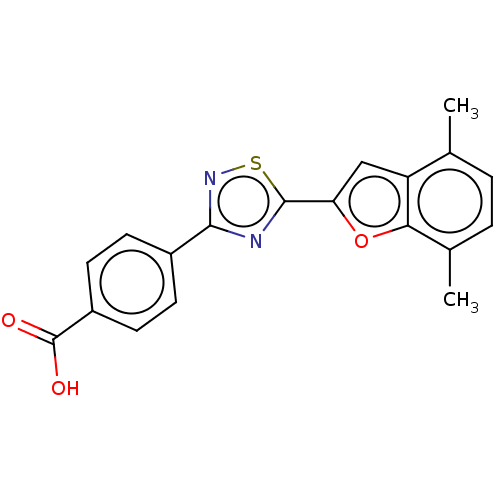
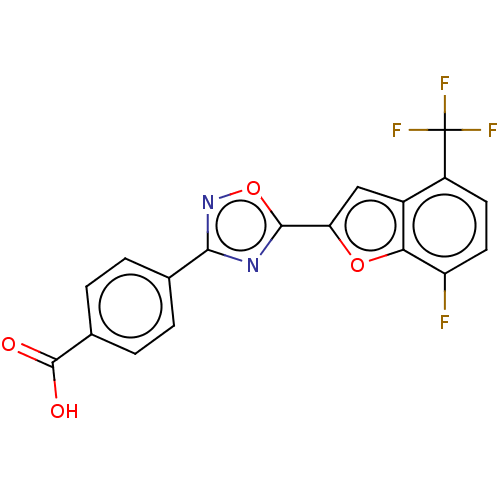
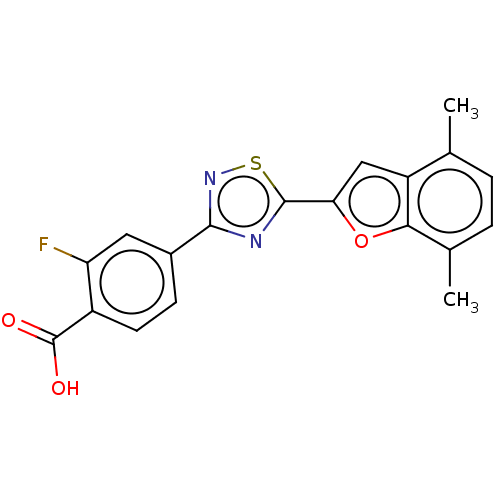
Affinity DataEC50: >0.00100nMAssay Description:Transcriptional activation of Retinoic acid receptor RAR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: >0.00100nMAssay Description:Transcriptional activation of Retinoic acid receptor RAR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: >0.00100nMAssay Description:Transcriptional activation of Retinoic acid receptor RAR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: >0.00100nMAssay Description:Transcriptional activation of Retinoic acid receptor RAR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: >0.00100nMAssay Description:Transcriptional activation of Retinoic acid receptor RAR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 0.180nMAssay Description:Agonist activity at human RARalpha expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.300nMAssay Description:Activity at human RARalpha ligand binding domain expressed in COS7 cells cotransfected with Gal4-DBD assessed as transcriptional activation after 16 ...More data for this Ligand-Target Pair
Affinity DataEC50: 0.600nMAssay Description:Activity at human RARalpha ligand binding domain expressed in COS7 cells cotransfected with Gal4-DBD assessed as transcriptional activation after 16 ...More data for this Ligand-Target Pair
Affinity DataEC50: 1nMAssay Description:Agonist activity at human RAR-alpha expressed in CHO-K1 cells by PathHunter assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1nMAssay Description:Transactivation of human Gal4-DBD-fused RARalpha-LBD expressed in HEK293T cells after 16 to 24 hrs by FRET based beta-lactamase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1.20nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 2nMAssay Description:Effective concentration for retinoic acid receptor RAR gamma transcriptional activationMore data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Transcriptional activation in CV-1 cells expressiing Retinoic acid receptor RAR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Agonist activity at RARalpha by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Transcriptional activation of Retinoic acid receptor RAR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Transcriptional activation of Retinoic acid receptor RAR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Transcriptional activation of retinoic acid receptor RAR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Transcriptional activation of Retinoic acid receptor RAR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Transcriptional activation for RAR alpha receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 5.5nMAssay Description:Retinoid nuclear receptor activity is associated with transduction of the non-visual physiologic, pharmacologic, and toxicologic retinoid signals tha...More data for this Ligand-Target Pair
Affinity DataEC50: 6nMAssay Description:Transcriptional activation in CV-1 cells expressiing Retinoic acid receptor RAR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 6.31nMAssay Description:Activity at RARalpha expressed in mouse NIH3T3 cells by R-SAT assayMore data for this Ligand-Target Pair
Affinity DataEC50: 7nMAssay Description:Compound was tested for binding affinity against retinoid X receptor using 5 nM of [3H]-9-cis-RA as a radioligand in baculovirus expressed receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 7nMAssay Description:Transcriptional activation in CV-1 cells expressing Retinoic acid receptor RAR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 8nMAssay Description:Binding affinity for retinoic acid receptor RAR gammaMore data for this Ligand-Target Pair
Affinity DataEC50: 8.20nMAssay Description:Transactivation of GST-tagged RARalpha LBD (unknown origin) assessed as fluorescein-labelled coactivator peptide recruitment measured after 4 hrs by ...More data for this Ligand-Target Pair
Affinity DataEC50: 9.40nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 11nMAssay Description:Transcriptional activation for RAR alpha receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 11nMAssay Description:Agonist activity at GAL4 DNA-binding domain-tagged RARalpha (unknown origin) ligand-binding domain expressed in human HG5LN cells incubated for 18 hr...More data for this Ligand-Target Pair
Affinity DataEC50: 12nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 14nMAssay Description:Transcriptional activation of Retinoic acid receptor RAR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 15nMAssay Description:Transcriptional activation for RAR alpha receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 15nMAssay Description:Transactivation of GST-tagged RARalpha LBD (unknown origin) assessed as fluorescein-labelled coactivator peptide recruitment measured after 4 hrs by ...More data for this Ligand-Target Pair
Affinity DataEC50: 16nMAssay Description:Transactivation of GST-tagged RARalpha LBD (unknown origin) assessed as fluorescein-labelled coactivator peptide recruitment measured after 4 hrs by ...More data for this Ligand-Target Pair
Affinity DataEC50: 16nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 17nMAssay Description:Activity at human RARalpha ligand binding domain expressed in COS7 cells cotransfected with Gal4-DBD assessed as transcriptional activation after 16 ...More data for this Ligand-Target Pair
Affinity DataEC50: 17nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 17nMAssay Description:Transactivation potency of the compound was determined for Retinoic acid receptor alpha; Not activeMore data for this Ligand-Target Pair
Affinity DataEC50: 18nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 19nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 19nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 20nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 21nMAssay Description:Transcriptional activation for RAR alpha receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 21nMAssay Description:Transcriptional activation of retinoic acid receptor RAR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 21nMAssay Description:Transcriptional activation of Retinoic acid receptor RAR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 21nMAssay Description:Transcriptional activation in CV-1 cells expressing retinoic acid receptor RAR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 22nMAssay Description:Agonist activity at GAL4 DNA-binding domain-tagged RARalpha (unknown origin) ligand-binding domain expressed in human HG5LN cells incubated for 18 hr...More data for this Ligand-Target Pair
Affinity DataEC50: 23nMAssay Description:Relative activity against Retinoic acid receptor RAR alpha compared to ATRAMore data for this Ligand-Target Pair
Affinity DataEC50: 23nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 24nMAssay Description:Transcriptional activation for RAR alpha receptorMore data for this Ligand-Target Pair





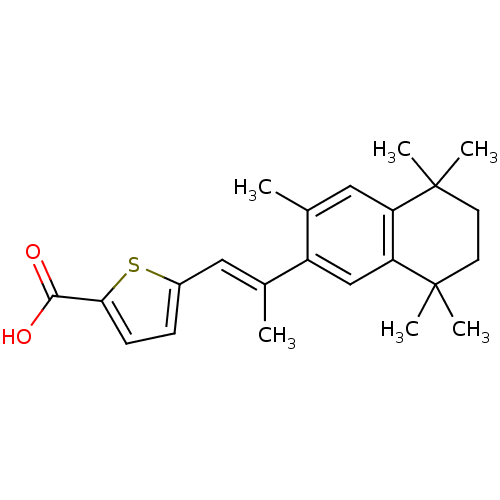
 3D Structure (crystal)
3D Structure (crystal)