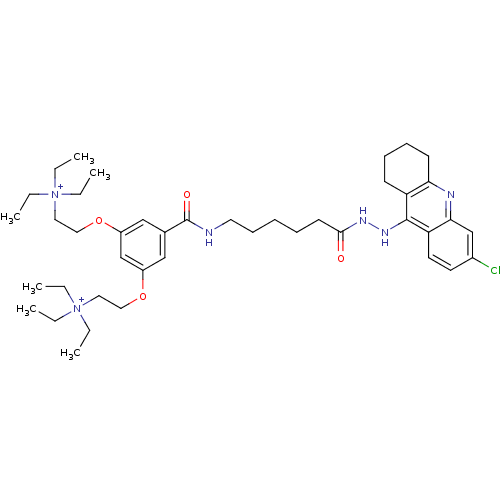
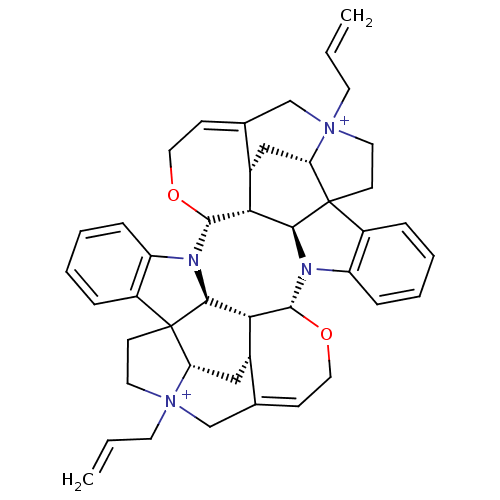
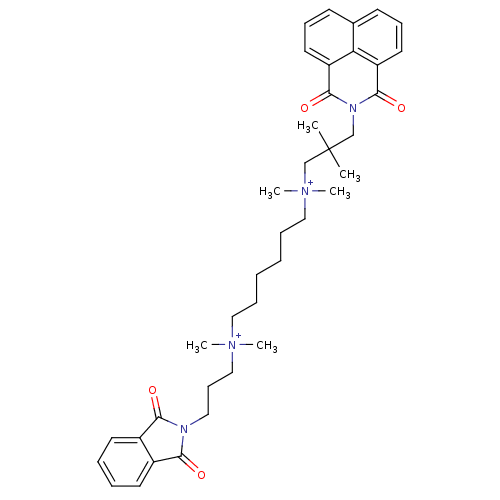
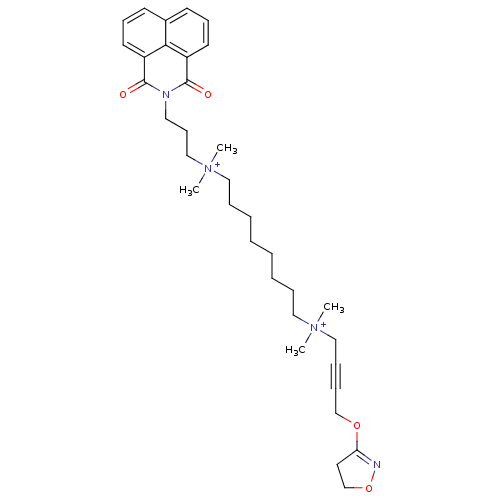
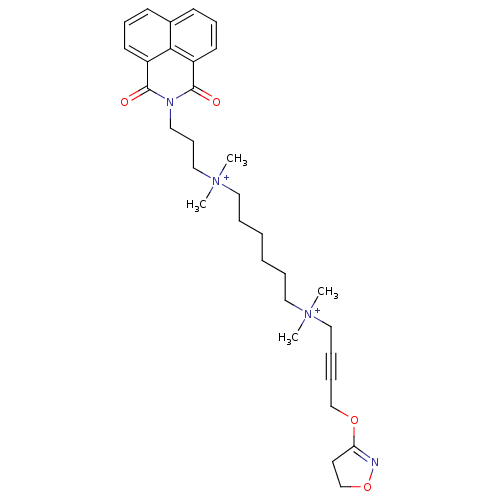
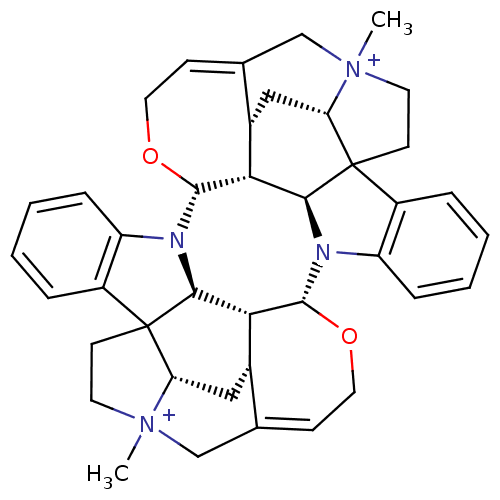
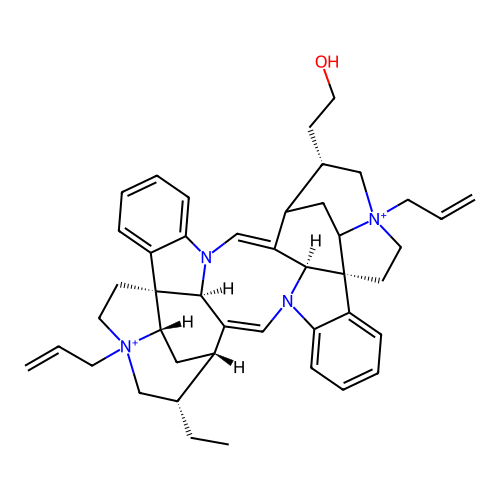
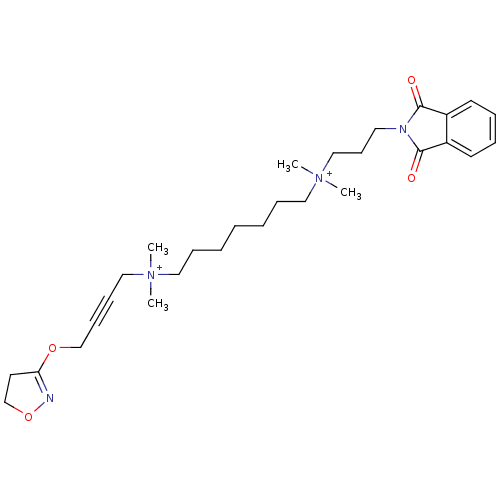
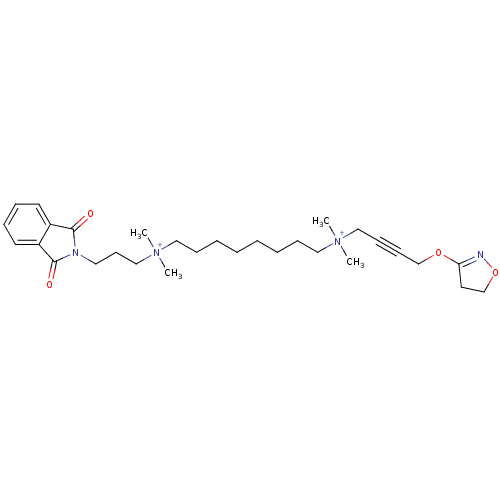
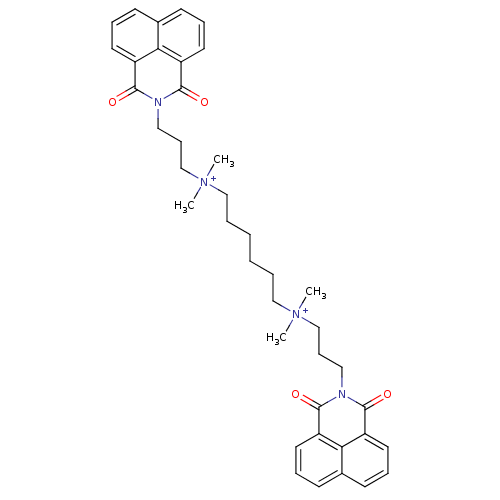
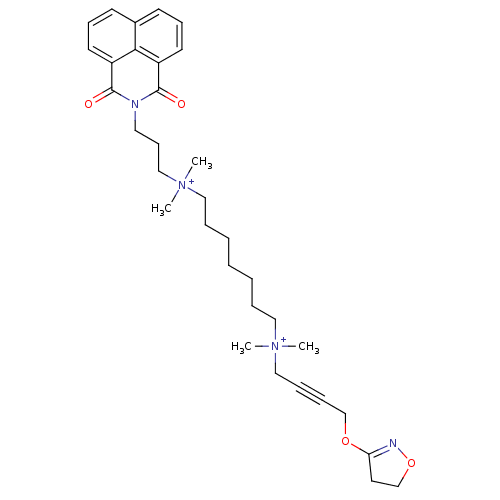
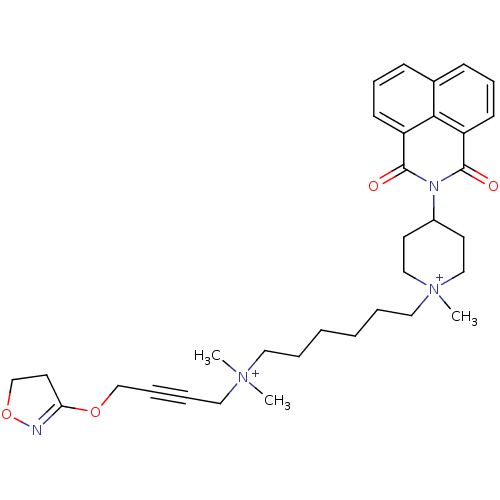
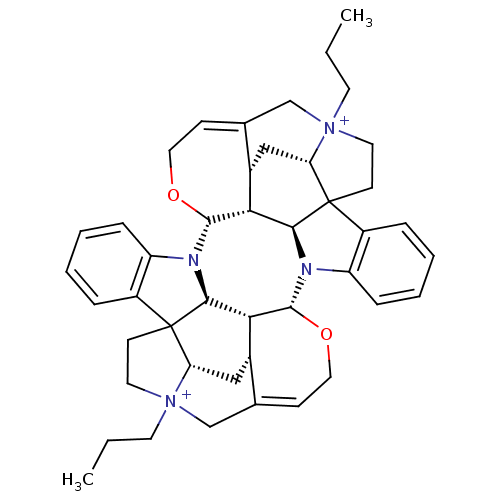
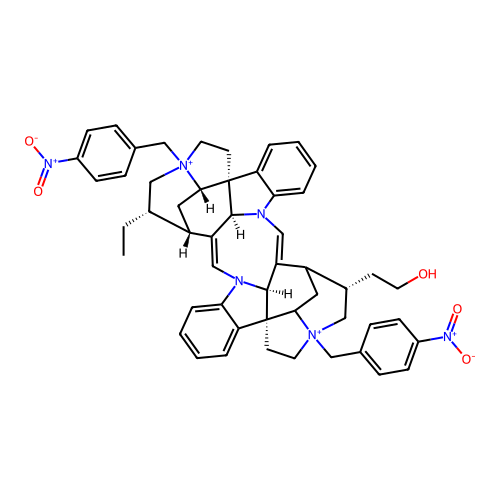
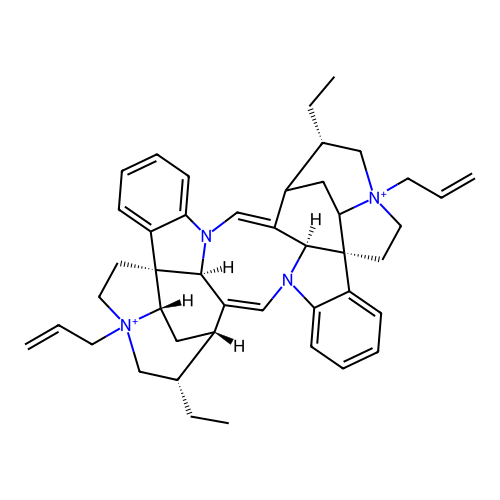
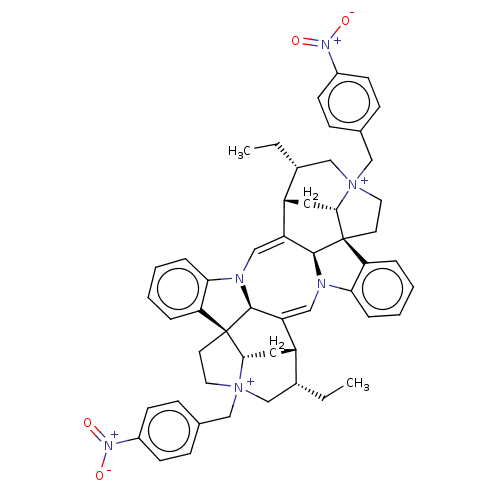
Affinity DataEC50: 0.0794nMAssay Description:Agonist activity at muscarinic M2 receptor in albino guinea pig left atrium assessed as stimulation of electrically-induced responseMore data for this Ligand-Target Pair
Affinity DataEC50: 0.166nMAssay Description:Agonist activity at muscarinic M3 receptor in albino guinea pig ileum assessed as stimulation of electrically-induced responseMore data for this Ligand-Target Pair
Affinity DataEC50: 0.900nMAssay Description:Inhibition of [3H]NMS dissociation from porcine muscarinic M2 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 1nMAssay Description:Inhibition of [3H]NMS dissociation from porcine muscarinic M2 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 1.30nMAssay Description:Inhibition of [3H]NMS dissociation from porcine muscarinic M2 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 1.40nMAssay Description:Inhibition of [3H]NMS dissociation from porcine muscarinic M2 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 1.5nMAssay Description:Inhibition of [3H]NMS dissociation from porcine muscarinic M2 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 2nMAssay Description:Displacement of [3H]NMS from pig muscarinic M2 receptor after 120 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 3nMAssay Description:Allosteric inhibition of [3H]-NMS (N-methylscopolamine) dissociation from porcine cardiac Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Allosteric inhibition of [3H]-NMS (N-methylscopolamine) dissociation from porcine cardiac Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
Affinity DataEC50: 4.40nMAssay Description:Effective concentration required to inhibit dissociation of [3H]NMS from porcine heart ventricles muscarinic acetylcholine receptor M2.More data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Inhibition of [3H]NMS dissociation from porcine muscarinic M2 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 7.10nMAssay Description:Agonist activity at muscarinic M2 receptor in albino guinea pig left atrium assessed as stimulation of electrically-induced responseMore data for this Ligand-Target Pair
Affinity DataEC50: 7.60nMAssay Description:Effective concentration required to inhibit dissociation of [3H]NMS from porcine heart ventricles muscarinic acetylcholine receptor M2.More data for this Ligand-Target Pair
Affinity DataEC50: 7.80nMAssay Description:Agonist activity at muscarinic M2 receptor in albino guinea pig left atrium assessed as stimulation of electrically-induced responseMore data for this Ligand-Target Pair
Affinity DataEC50: 7.80nMAssay Description:Effective concentration required to inhibit dissociation of [3H]NMS from porcine heart ventricles muscarinic acetylcholine receptor M2.More data for this Ligand-Target Pair
Affinity DataEC50: 8nMAssay Description:Allosteric inhibition of [3H]-NMS (N-methylscopolamine) dissociation from porcine cardiac Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
Affinity DataEC50: 8nMAssay Description:Displacement of [3H]NMS from pig muscarinic M2 receptor after 120 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 8.5nMAssay Description:Effective concentration required to inhibit dissociation of [3H]NMS from porcine heart ventricles muscarinic acetylcholine receptor M2.More data for this Ligand-Target Pair
Affinity DataEC50: 11nMAssay Description:Agonist activity at muscarinic M3 receptor in albino guinea pig ileum assessed as stimulation of electrically-induced responseMore data for this Ligand-Target Pair
Affinity DataEC50: 11nMAssay Description:Displacement of [3H]NMS from pig muscarinic M2 receptor after 120 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 12nMAssay Description:Displacement of [3H]NMS from pig muscarinic M2 receptor after 120 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 12nMAssay Description:Displacement of [3H]NMS from pig muscarinic M2 receptor after 120 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 12nMAssay Description:Agonist activity at muscarinic M2 receptor in albino guinea pig left atrium assessed as stimulation of electrically-induced responseMore data for this Ligand-Target Pair
Affinity DataEC50: 14nMAssay Description:Agonist activity at muscarinic M2 receptor in albino guinea pig left atrium assessed as stimulation of electrically-induced responseMore data for this Ligand-Target Pair
Affinity DataEC50: 14nMAssay Description:Effective concentration required to inhibit dissociation of [3H]NMS from porcine heart ventricles muscarinic acetylcholine receptor M2.More data for this Ligand-Target Pair
Affinity DataEC50: 14nMAssay Description:Effective concentration required to inhibit dissociation of [3H]NMS from porcine heart ventricles muscarinic acetylcholine receptor M2.More data for this Ligand-Target Pair
Affinity DataEC50: 19nMAssay Description:Agonist activity at muscarinic M2 receptor in albino guinea pig left atrium assessed as stimulation of electrically-induced responseMore data for this Ligand-Target Pair
Affinity DataEC50: 19nMAssay Description:Effective concentration required to inhibit dissociation of [3H]NMS from porcine heart ventricles muscarinic acetylcholine receptor M2.More data for this Ligand-Target Pair
Affinity DataEC50: 21nMAssay Description:Agonist activity at muscarinic M3 receptor in albino guinea pig ileum assessed as stimulation of electrically-induced responseMore data for this Ligand-Target Pair
Affinity DataEC50: 21nMAssay Description:Agonist activity at muscarinic M2 receptor in albino guinea pig left atrium assessed as stimulation of electrically-induced responseMore data for this Ligand-Target Pair
Affinity DataEC50: 25nMAssay Description:Effective concentration required to inhibit dissociation of [3H]NMS from porcine heart ventricles muscarinic acetylcholine receptor M2.More data for this Ligand-Target Pair
Affinity DataEC50: 30nMAssay Description:Allosteric inhibition of [3H]-NMS (N-methylscopolamine) dissociation from porcine cardiac Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
Affinity DataEC50: 32nMAssay Description:Displacement of [3H]NMS from pig muscarinic M2 receptor after 120 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 32nMAssay Description:Displacement of [3H]NMS from pig muscarinic M2 receptor after 120 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 36nMAssay Description:Effective concentration required to inhibit dissociation of [3H]NMS from porcine heart ventricles muscarinic acetylcholine receptor M2.More data for this Ligand-Target Pair
Affinity DataEC50: 36nMAssay Description:Effective concentration required to inhibit dissociation of [3H]NMS from porcine heart ventricles muscarinic acetylcholine receptor M2.More data for this Ligand-Target Pair
Affinity DataEC50: 36nMAssay Description:Displacement of [3H]NMS from pig muscarinic M2 receptor after 120 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 36nMAssay Description:Displacement of [3H]NMS from pig muscarinic M2 receptor after 120 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 38nMAssay Description:Agonist activity at muscarinic M2 receptor in albino guinea pig left atrium assessed as stimulation of electrically-induced responseMore data for this Ligand-Target Pair
Affinity DataEC50: 45nMAssay Description:Ability to dissociate radioligand [3H]NMS from muscarinic acetylcholine receptor M2 of porcine heartMore data for this Ligand-Target Pair
Affinity DataEC50: 47nMAssay Description:Effective concentration required to inhibit dissociation of [3H]NMS from porcine heart ventricles muscarinic acetylcholine receptor M2.More data for this Ligand-Target Pair
Affinity DataEC50: 47nMAssay Description:Agonist activity at muscarinic M3 receptor in albino guinea pig ileum assessed as stimulation of electrically-induced responseMore data for this Ligand-Target Pair
Affinity DataEC50: 49nMAssay Description:Displacement of [3H]NMS from pig muscarinic M2 receptor after 120 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 49nMAssay Description:Displacement of [3H]NMS from pig muscarinic M2 receptor after 120 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 50nMAssay Description:Agonist activity at muscarinic M3 receptor in albino guinea pig ileum assessed as stimulation of electrically-induced responseMore data for this Ligand-Target Pair
Affinity DataEC50: 52nMAssay Description:Effective concentration required to inhibit dissociation of [3H]NMS from porcine heart ventricles muscarinic acetylcholine receptor M2.More data for this Ligand-Target Pair
Affinity DataEC50: 55nMAssay Description:Effective concentration required to inhibit dissociation of [3H]NMS from porcine heart ventricles muscarinic acetylcholine receptor M2.More data for this Ligand-Target Pair
Affinity DataEC50: 60nMAssay Description:Ability to dissociate radioligand [3H]NMS from muscarinic acetylcholine receptor M2 of porcine heartMore data for this Ligand-Target Pair
Affinity DataEC50: 63nMAssay Description:Ability to dissociate radioligand [3H]NMS from muscarinic acetylcholine receptor M2 of porcine heartMore data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)