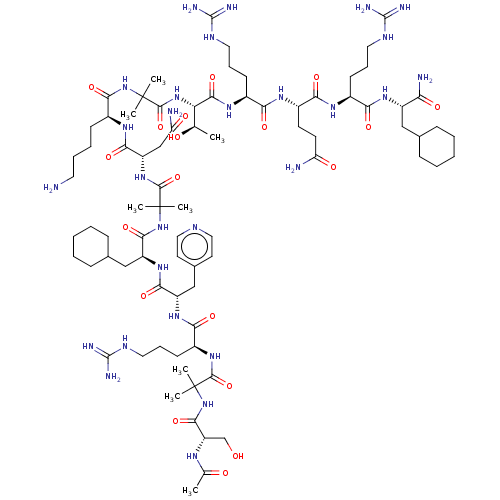
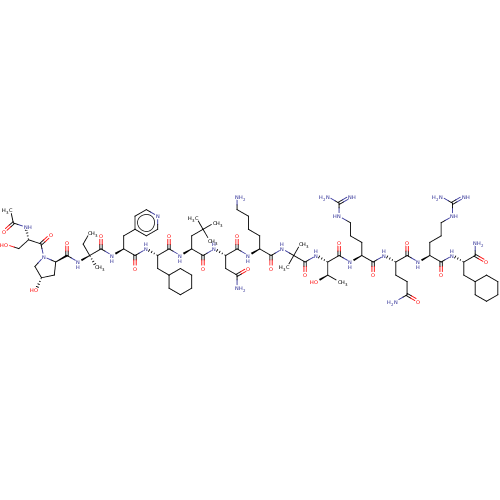
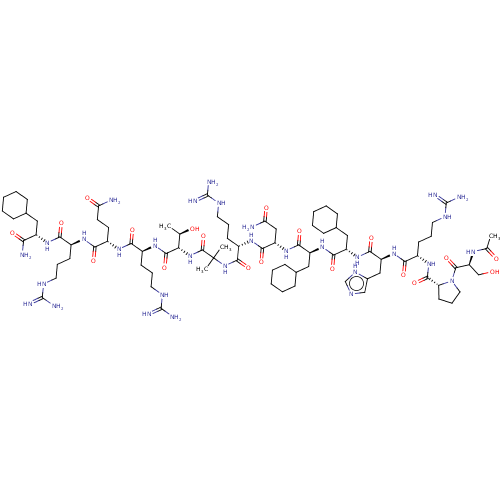
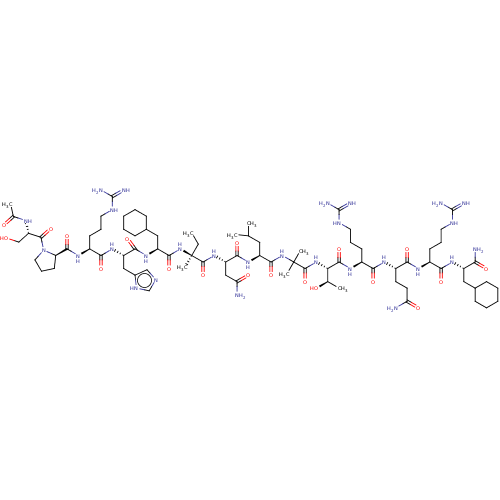
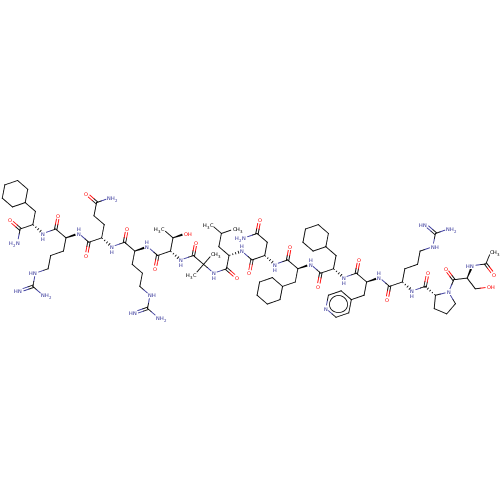
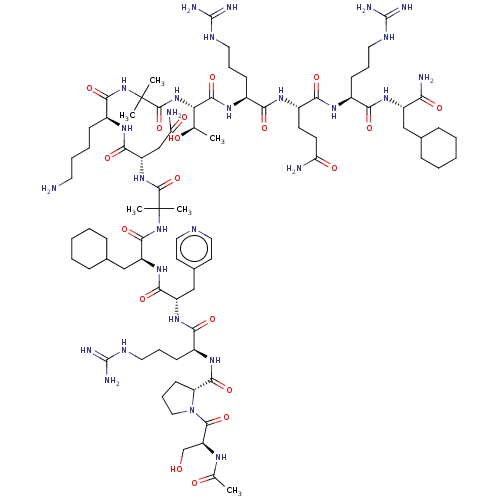
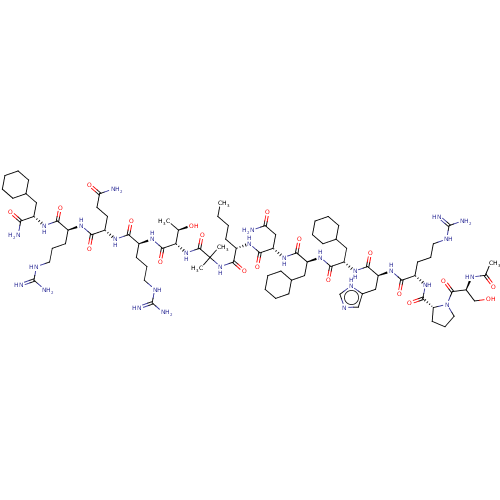
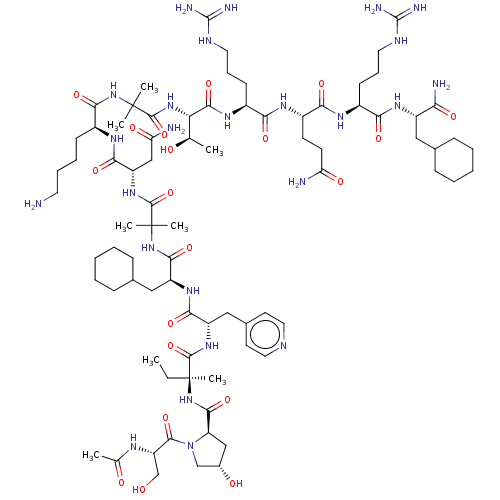
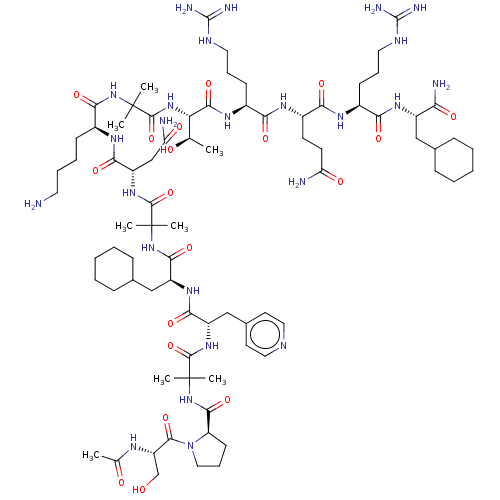
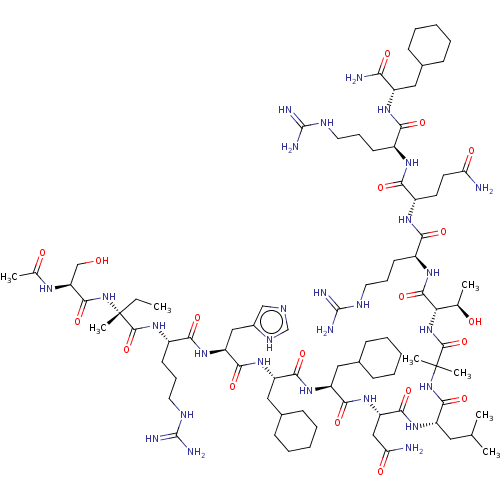
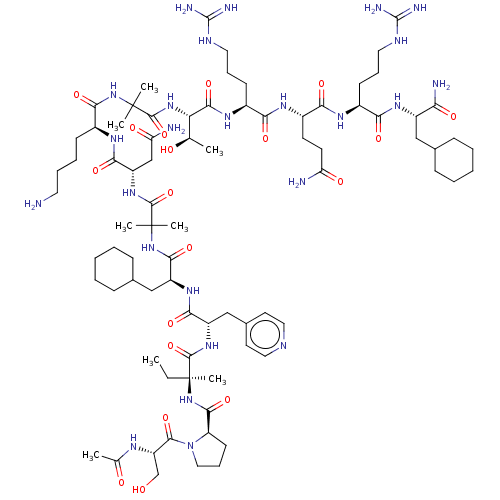
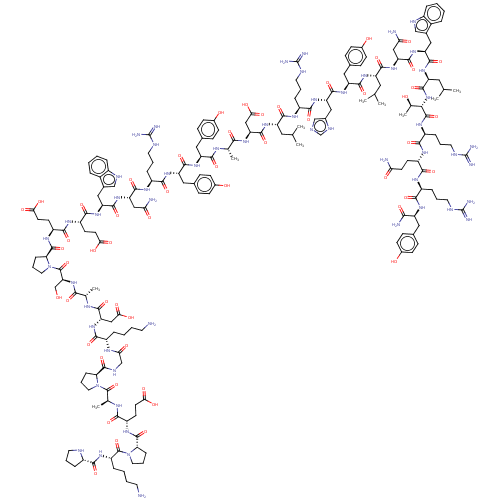
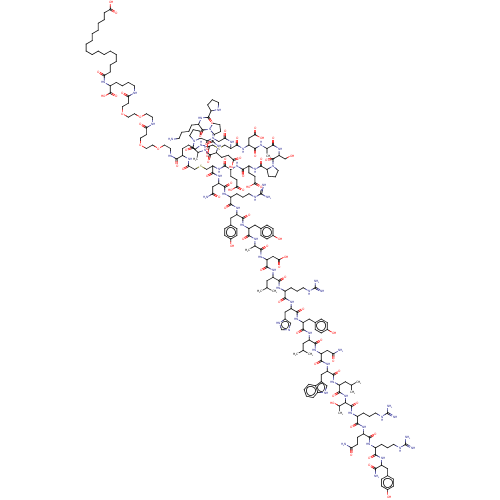
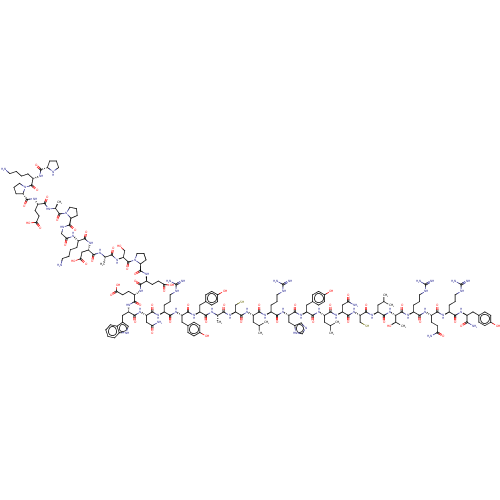
Affinity DataEC50: 0.0380nMAssay Description:Agonist activity at rat neuropeptide Y2 receptor by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0631nMAssay Description:Positive allosteric modulation of human C-terminal eYFP-fused human NY2 receptor expressed in African green monkey COS7 cells co-expressing delta6Gal...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0680nMAssay Description:Agonist activity at human Y2 receptor expressed in CHO cell membranes after 120 mins by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.110nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.110nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.130nMAssay Description:Agonist activity at human NPY2R expressed in CHO cells assessed as inhibition of forskolin-induced intracellular cAMP accumulation incubated for 30 m...More data for this Ligand-Target Pair
Affinity DataEC50: 0.130nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.130nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.130nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.140nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.150nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.160nMAssay Description:Agonist activity at human Y2 receptor expressed in CHO cell membranes after 120 mins by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.160nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.170nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.170nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.170nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.180nMAssay Description:Agonist activity at human NPY2R expressed in CHO cells assessed as inhibition of forskolin-induced intracellular cAMP accumulation incubated for 30 m...More data for this Ligand-Target Pair
Affinity DataEC50: 0.180nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.180nMAssay Description:Agonist activity at human NPY2R expressed in CHO cells assessed as inhibition of forskolin-induced intracellular cAMP accumulation incubated for 30 m...More data for this Ligand-Target Pair
Affinity DataEC50: 0.180nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.190nMAssay Description:Agonist activity at human Y2 receptor expressed in CHO cell membranes after 120 mins by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at human NPY2R expressed in HEK293 cells co-transfected with CRE-luciferase incubated for 24 hrs in presence of 10% FBS by One-Glo l...More data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at human Y2 receptor expressed in CHO cell membranes after 120 mins by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at human Y2 receptor expressed in CHO cell membranes after 120 mins by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at human Y2 receptor expressed in CHO cell membranes after 120 mins by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Displacement of [125I]-PYY from human Y2R expressed in CHO cell membranes after 60 mins by liquid scintillation methodMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.210nMAssay Description:Agonist activity at human Y2 receptor expressed in CHO cell membranes after 120 mins by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.210nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.210nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.280nMAssay Description:Agonist activity at human Y2 receptor expressed in CHO cell membranes after 120 mins by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.280nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.280nMAssay Description:Agonist activity at human Y2R expressed in CHO cell membranes assessed as [35S]GTPgammaS binding after 120 mins by liquid scintillation methodMore data for this Ligand-Target Pair
Affinity DataEC50: 0.280nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.280nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.290nMAssay Description:Agonist activity at human NPY2R expressed in CHO cells assessed as inhibition of forskolin-induced intracellular cAMP accumulation incubated for 30 m...More data for this Ligand-Target Pair
Affinity DataEC50: 0.300nMAssay Description:Agonist activity at human NPY2R expressed in HEK293 cells co-transfected with CRE-luciferase incubated for 24 hrs in presence of 10% FBS by One-Glo l...More data for this Ligand-Target Pair
Affinity DataEC50: 0.300nMAssay Description:Agonist activity at human NPY2 receptor expressed in human KAN-TS cells by [35S]GTPgammaS incorporation assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.300nMAssay Description:Agonist activity at human NPYY2 receptor expressed in HEK293 cells assessed as decrease in isoproterenol-induced cAMP level measured every 30 secs fo...More data for this Ligand-Target Pair
Affinity DataEC50: 0.320nMAssay Description:Agonist activity at human NPY2R expressed in CHO cells assessed as inhibition of forskolin-induced intracellular cAMP accumulation incubated for 30 m...More data for this Ligand-Target Pair
Affinity DataEC50: 0.320nMAssay Description:Agonist activity at human NPY2R expressed in CHO cells assessed as inhibition of forskolin-induced intracellular cAMP accumulation incubated for 30 m...More data for this Ligand-Target Pair
Affinity DataEC50: 0.330nMAssay Description:Agonist activity at human Y2 receptor expressed in CHO cell membranes after 120 mins by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.350nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.398nMAssay Description:Agonist activity at human neuropeptide Y Y2 receptor expressed in COS-7 cells cotransfected with chimeric Gi/q protein assessed as inositol phosphate...More data for this Ligand-Target Pair
Affinity DataEC50: 0.400nMAssay Description:Agonist activity at human neuropeptide Y Y2 receptor expressed in COS-7 cells cotransfected with chimeric Gi/q protein assessed as inositol phosphate...More data for this Ligand-Target Pair
Affinity DataEC50: 0.400nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.410nMAssay Description:Agonist activity at human NPY2R expressed in CHO cells assessed as inhibition of forskolin-induced intracellular cAMP accumulation incubated for 30 m...More data for this Ligand-Target Pair
Affinity DataEC50: 0.410nMAssay Description:Agonist activity at human neuropeptide Y2 receptor expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 12...More data for this Ligand-Target Pair
Affinity DataEC50: 0.410nMAssay Description:Agonist activity at human Y2 receptor expressed in CHO cell membranes after 120 mins by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair